Menu
Physics Lesson 19.4.1 - De Broglie Wave and De Broglie Relation
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on De Broglie Wave and De Broglie Relation, this is the first lesson of our suite of physics lessons covering the topic of De Broglie Wave, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
De Broglie Wave and De Broglie Relation
In 1924, when working for his PhD thesis, Louis De Broglie presented his hypothesis on the wave nature of electron. By generalizing the particle-wave dualism, he supported the idea that not only EM radiation has a particle nature but the matter particles (electrons) have a wave nature as well.
By "wave nature of electron", De Broglie did not intend the radiation of any kind of special EM wave produced by electrons in motion or the transformation of electrons in any kind of sustainable matter wave. Rather, he supported the idea that "moving electrons have a corresponding kind of wave associated, whose wavelength is determined only by the electron momentum."
De Broglie was not able to explain the nature of this "electron wave" which we call "De Broglie wave", but he postulated the relationship between its wavelength and the impulse of electron. It is
Remark! Sometimes in quantum physics we used another constant instead of Planck constant h, known as the reduced Planck constant, ℏ. Its relationship with the Planck constant is
De Broglie applied the analogy with the corresponding equation used for photons. As we know, the two equations used for light waves are
Combining these two equation, we obtain
= h/λ
Thus,
From the last equation, it is clear that the De Broglie relation represents a generalization of the particle-wave dualism.
Example 1
Calculate the wavelength of De Broglie wave of an electron accelerated in a 100 V potential difference. Take the mass of electron m = 9.1 × 10-31 kg.
Solution 1
Initially, we find the momentum p of electron using its relationship with the kinetic energy. We have
= p2/2m
On the other hand, it is obvious that the kinetic energy of electron (assumed that initially it was at rest), is numerically equal to the work done by electric forces, i.e.
p2/2m = e ∙ ∆V
where e is the elementary charge of electron (e = 1.6 × 10-19 C). Hence, we obtain for the momentum of electron:
= √2 ∙ (9.1 × 10-31 kg) ∙ (1.6 × 10-19 C) ∙ (102 V)
= √29.12 × 10-48
= 5.396 × 10-24 kg ∙ m/s
Therefore, the wavelength of De Broglie wave is
= h/√2 ∙ m ∙ e ∙ ∆V
= 6.626 × 10-34 J ∙ s/5.396 × 10-24 kg ∙ m/s
= 1.23 × 10-10 m
= 0.123 nm
From this example, it is clear that the electron's wavelength is comparable to wavelengths of X-radiation of EM waves. This is why electronic beams incident on crystals gives diffract visibly and produce patterns similar to those produced by X-rays. In this sense, expressions like "electron has a wave nature" or "electron manifests wave behavior" are very meaningful.
Example 2
An electronic beam diffracts after passing through an a = 12 μm narrow gap and the diffraction pattern is observed on a fluorescent screen placed at a distance of L = 4 m from the gap, as shown in the figure.
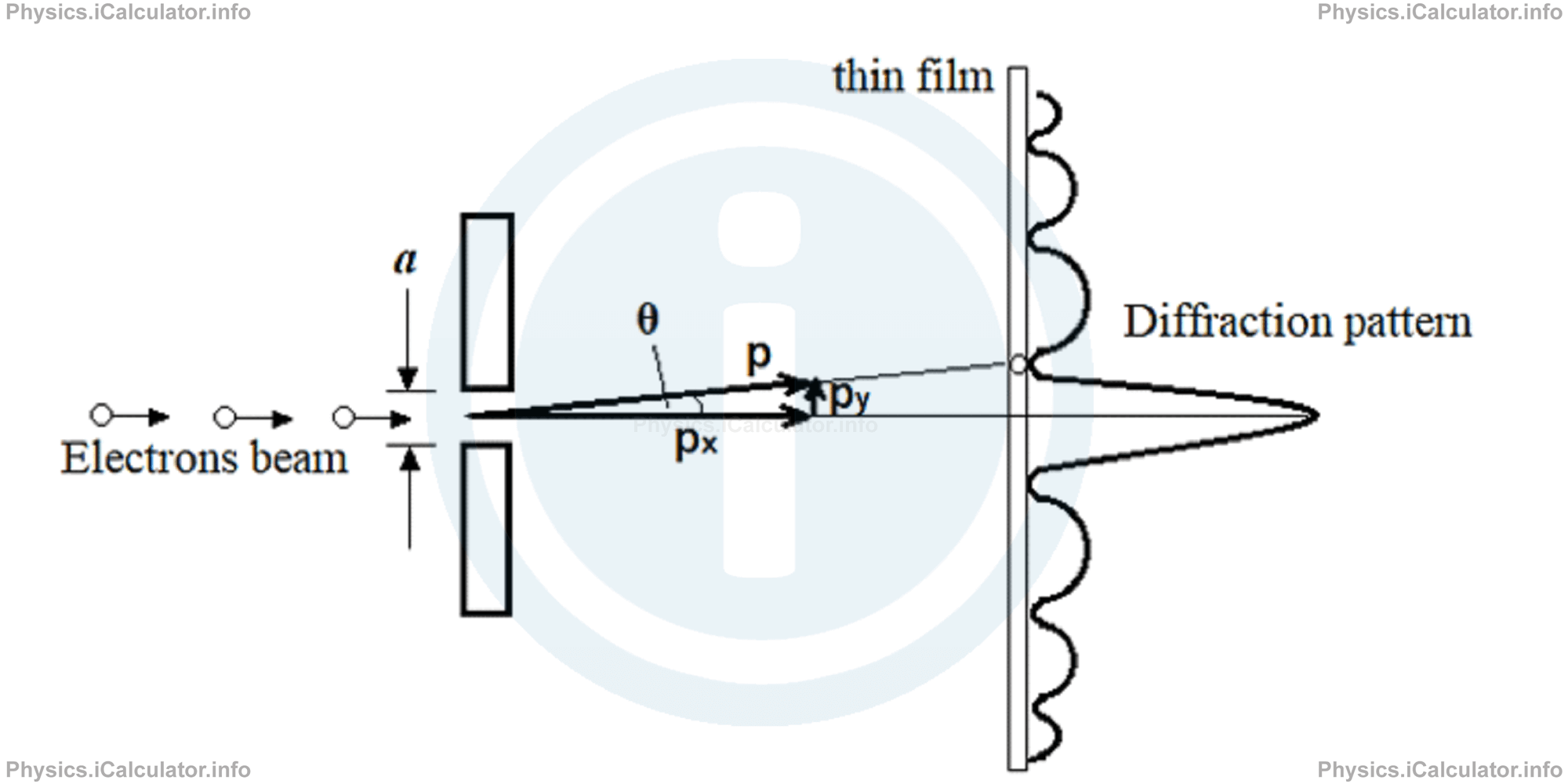
Calculate the wavelength of De Broglie wave for electrons, given that measurement gave the value of Δy = 0.2 mm for the width of central maximum. Refer to tutorial> 12.4 for the formula of diffraction.
Solution 2
Clues:
a = 12 μm = 12 × 10-6 m = 1.2 × 10-5 m
L = 4 m
Δy = 0.2 mm = 2 × 10-4 m
λ = ?
The diffraction pattern indicates the density of electrons distribution on the thin film acting as a screen. It is obvious that most electrons are incident on the central maximum as the curve is higher at this section. In other words, the x-coordinate of diffraction pattern is proportional to the number of electrons incident on that specific section of the screen.
We know from tutorial>tutorial 12.4 that the condition for a maximum to occur is
where a is the thickness of the gap (slit), λ is the wavelength of the incident EM radiation and N is the order of diffraction (here N = 1 as we are interested only for the central maximum).
The angle θ is very small (we reach in this conclusion by comparing the vertical displacement Δy, which acts as a vertical component for the angle θ, to the distance L of the gap from the screen which here acts as a horizontal component of the angle θ). Thus, we can write (based on the trigonometric approximation you know from math):
= ∆y/2/L
= ∆y/2L
Thus, comparing the two above equations for sin θ, we obtain
Hence, we obtain for De Broglie wavelength λ:
= (1.2 × 10-5 m) ∙ (2 × 10-4 m)/2 ∙ (4 m)
= 0.3 × 10-9 m
= 0.3 nm
You have reached the end of Physics lesson 19.4.1 De Broglie Wave and De Broglie Relation. There are 2 lessons in this physics tutorial covering De Broglie Wave, you can access all the lessons from this tutorial below.
More De Broglie Wave Lessons and Learning Resources
Whats next?
Enjoy the "De Broglie Wave and De Broglie Relation" physics lesson? People who liked the "De Broglie Wave lesson found the following resources useful:
- Wave Relation Feedback. Helps other - Leave a rating for this wave relation (see below)
- Modern Physics Physics tutorial: De Broglie Wave. Read the De Broglie Wave physics tutorial and build your physics knowledge of Modern Physics
- Modern Physics Revision Notes: De Broglie Wave. Print the notes so you can revise the key points covered in the physics tutorial for De Broglie Wave
- Modern Physics Practice Questions: De Broglie Wave. Test and improve your knowledge of De Broglie Wave with example questins and answers
- Check your calculations for Modern Physics questions with our excellent Modern Physics calculators which contain full equations and calculations clearly displayed line by line. See the Modern Physics Calculators by iCalculator™ below.
- Continuing learning modern physics - read our next physics tutorial: Electromagnetic Wave Packet. The Uncertainty Principle
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "De Broglie Wave" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Modern Physics Calculators by iCalculator™
- Characteristic Em Wavelength Calculator
- De Broglie Wave Calculator
- Rayleigh Jeans Relation Calculator
- Energy Of Photons Calculator
- Intensity Photoelectric Effect Calculator
- Kinetic Photoelectric Effect Calculator
- Light Pressure Calculator
- Radiation Black Body Calculator
- Stopping Voltage Photoelectric Effect Calculator
- Uncertainty Calculator
- Wave Width Calculator
- Scattered Radiation Compton Effect Calculator
- De Broglie Packet Calculator