Menu
Physics Lesson 19.1.6 - The Ultraviolet Catastrophe and Planck's Hypothesis
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on The Ultraviolet Catastrophe and Planck's Hypothesis, this is the sixth lesson of our suite of physics lessons covering the topic of Thermal Radiation. Photon as the Quantum of Light, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
The Ultraviolet Catastrophe and Planck's Hypothesis
All attempts of scientists during the XIX century in finding a formula that corresponds to the graph of spectral emissivity, failed. This is because they relied on the classical approach, which stresses the continuous nature of electromagnetic radiation emitted by atoms and molecules. Based on this hypothesis, two scientists - Rayleigh and Jeans - found the mathematical expression below for the emission ability of a black body:
The graph below shows the curve found experimentally (the solid curve) and the theoretical curve obtained through the formula of Rayleigh-Jeans.
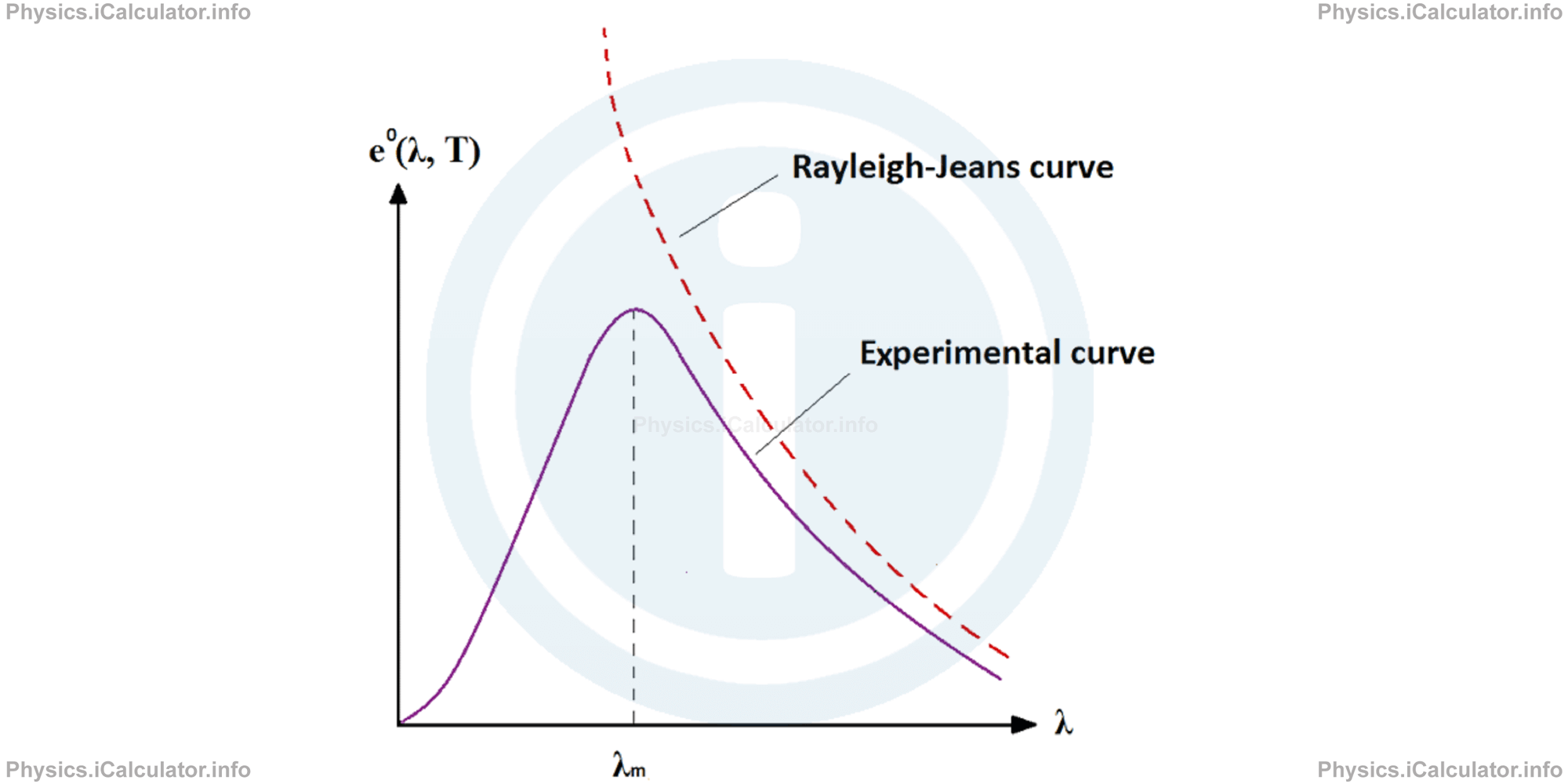
From the graph is evident that for long wavelength the curve obtained from the Rayleigh-Jeans formula represents a good fit for the experimental findings but for short wavelengths, this curve deflects too much from the curve found experimentally. Moreover, the emission ability of objects points towards infinity when wavelength points towards zero.
It was an absurdity to accept the idea that object with finite dimensions could have an infinite emission ability and they are able to radiate infinitely large amounts of energy in every second. This nonsense represents a notable failure of the classical theory of thermal radiation, which is known in the history of physics as the "ultraviolet catastrophe".
In 1900, Max Planck overcame this handicap by proposing a new hypothesis. According to him, the radiation emitted from atoms and molecules do not occur in a continuous way but with interruptions and in very small portions called quanta (quantum in singular). Moreover, the energy E of a quantum of light (known as photon - the particle of light) is proportional to the frequency of radiation, i.e.
where h (which is a pseudo-letter; it is not the traditional h of our alphabet) represents the Planck constant. It has the value of 6.626 × 10-34 J/s.
Relying on his hypothesis on the quantum nature of light, Max Planck managed to find theoretically the true shape of the spectral emissivity curve for a black body and to explain the experimental findings (laws) of Stephan-Boltzmann and Wien. The compliance with the experimental data was complete. With the Planck hypothesis, a new era of modern physics represented by the quantum physics, did start. This new branch of physics today stands at the forefront of scientific development and technology.
The above equation represents the fundamental equation of quantum theory in Modern Physics.
Example 1
Stefan and Boltzmann found experimentally the value of constant σ that bears their name. As stated earlier, it is σ = 5.67 × 10-8 J/K4m2s, while Planck found theoretically that this constant is related to the other three universal constants h, k and c (Planck constant, Boltzmann constant and light speed in vacuum respectively) through the expression
Based on the above formula, calculate the value of Planck's constant h giving that k = 1.38 × 10-23 J/mol·K and c = 3 × 108 m/s.
Solution 1
This simple exercise involves only calculations. Thus, after rearranging, we obtain for the Planck's constant h:
h = ∛ 2π5 ∙ k4/15c2 ∙ σ
= ∛ 2 ∙ (3.14)5 ∙ (1.38 × 10-23 J/mol ∙ K)4/15 ∙ (3 × 108 m/s)2 ∙ (5.67 × 10-8 J/K4 ∙ m2 ∙ s)
= ∛ 289.24 × 10-102 J3/s3
= 6.613×10-34 J/s
You have reached the end of Physics lesson 19.1.6 The Ultraviolet Catastrophe and Planck's Hypothesis. There are 6 lessons in this physics tutorial covering Thermal Radiation. Photon as the Quantum of Light, you can access all the lessons from this tutorial below.
More Thermal Radiation. Photon as the Quantum of Light Lessons and Learning Resources
Whats next?
Enjoy the "The Ultraviolet Catastrophe and Planck's Hypothesis" physics lesson? People who liked the "Thermal Radiation. Photon as the Quantum of Light lesson found the following resources useful:
- Max Planck Feedback. Helps other - Leave a rating for this max planck (see below)
- Modern Physics Physics tutorial: Thermal Radiation. Photon as the Quantum of Light. Read the Thermal Radiation. Photon as the Quantum of Light physics tutorial and build your physics knowledge of Modern Physics
- Modern Physics Revision Notes: Thermal Radiation. Photon as the Quantum of Light. Print the notes so you can revise the key points covered in the physics tutorial for Thermal Radiation. Photon as the Quantum of Light
- Modern Physics Practice Questions: Thermal Radiation. Photon as the Quantum of Light. Test and improve your knowledge of Thermal Radiation. Photon as the Quantum of Light with example questins and answers
- Check your calculations for Modern Physics questions with our excellent Modern Physics calculators which contain full equations and calculations clearly displayed line by line. See the Modern Physics Calculators by iCalculator™ below.
- Continuing learning modern physics - read our next physics tutorial: The Photoelectric Effect
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Thermal Radiation. Photon as the Quantum of Light" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Modern Physics Calculators by iCalculator™
- Characteristic Em Wavelength Calculator
- De Broglie Wave Calculator
- Rayleigh Jeans Relation Calculator
- Energy Of Photons Calculator
- Intensity Photoelectric Effect Calculator
- Kinetic Photoelectric Effect Calculator
- Light Pressure Calculator
- Radiation Black Body Calculator
- Stopping Voltage Photoelectric Effect Calculator
- Uncertainty Calculator
- Wave Width Calculator
- Scattered Radiation Compton Effect Calculator
- De Broglie Packet Calculator