Menu
Physics Lesson 19.2.2 - Einstein's Equation of Photoelectric Effect. The Particle Nature of Light
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Einstein's Equation of Photoelectric Effect. The Particle Nature of Light, this is the second lesson of our suite of physics lessons covering the topic of The Photoelectric Effect, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Einstein's Equation of Photoelectric Effect. The Particle Nature of Light
The phenomenon of photoelectric effect was explained by Einstein in 1905, who accepted as true the Planck's hypothesis on the discrete nature of light. Einstein elaborated further this idea by asserting that light is emitted, propagated and absorbed in specific portions (quanta) of energy. He supported the idea of the discrete nature of light and that light is composed by microscopic particles (photons) having the energy Ephoton = h · f and impulse p = E / c.
Here, c represents the speed of light particles or photons. Einstein considered the proton-electron interaction to explain the photoelectric effect and he applied the law of conservation of energy for this. Thus, the electron near the metal surface "absorbs" the photon and "consumes" part of photons energy (E = h · f) during its detachment from metal. This energy is known as Work Function, Φ and represent the minimum work necessary to detach the electron from metal. When the energy of incident photon absorbed by the electron during the photoelectric effect is greater than the work function, the electron gains kinetic energy and thus, it can escape from metal. In this way, the Einstein's Equation of Photoelectric Effect derived from the law of conservation of energy, is
or
The table below shows the work function in electronvolts, eV (1eV = 1.6 × 10-19 J) for a number of metals.
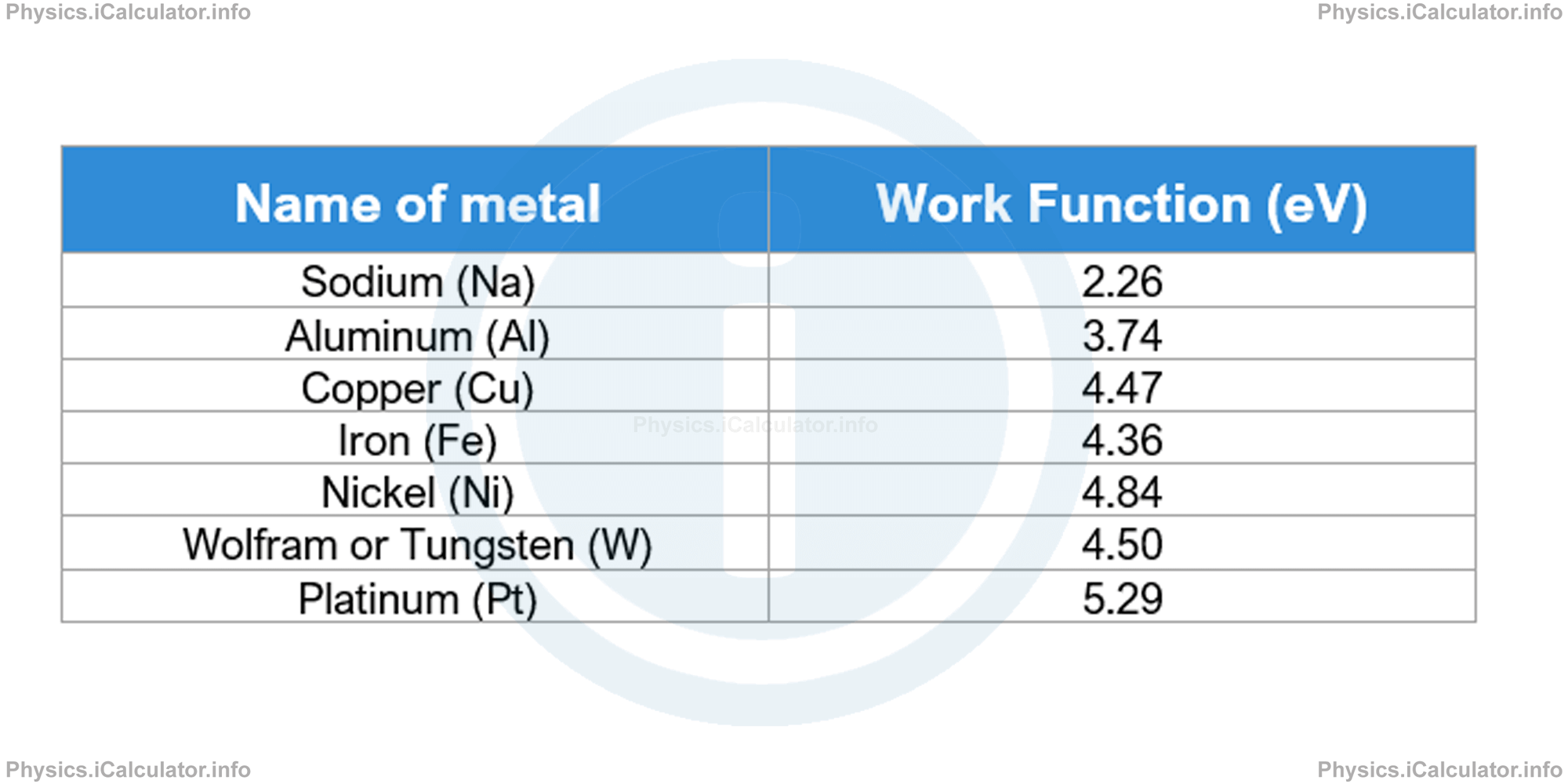
It is an interesting fact that Einstein won the Nobel Prize for explaining the phenomenon of photoelectric effect but did not win any Nobel Prize for his discoveries in relativity. This is because nobody from the Nobel Prize committee was able to understand in-depth his idea on relativity at that time.
Example 1
Calculate the wavelength of photon needed to make an electron move at 6 × 106 m/s towards the anode during the photoelectric effect. The cathode is made of tungsten. Take me ≈ 9.1 × 10-31 kg.
Solution 1
From the table above we see that the work function of tungsten is
= 4.50 ∙ 1.6 × 10-19 J
= 7.2 × 10-19 J
First, we must find the frequency of the incident photon, fph. From the Einstein's Equation of Photoelectric Effect, we have
fph = Φ + me ∙ ve2/2/h
= (7.2 × 10-19 J) + (9.1 × 10-31 kg) ∙ (6 × 106 m/s)2/2/6.626 × 10-34 J ∙ s
= 2.58 × 1016 Hz
Giving that for all EM waves we have
where c = 3 × 108 m/s, we obtain for the wavelength λph of the incident photon:
= 3 × 108 m/s/2.58 × 1016 Hz
= 1.163 × 10-8 m
= 11.63 nm (UV radiation)
When the electron-photon interaction occurs at the surface of metal, the kinetic energy resulting from the photoelectric effect has a higher value than when this interaction occurs inside the metal. This is because electron transmits some of its kinetic energy to the metal atoms during collisions taking place when it in on the way to move to the metal surface. The kinetic energy of electron in Einstein's Equation of Photoelectric Effect represents the maximum kinetic energy of electron, i.e. when applying this equation we assume the electron is on the surface of metal, not inside it.
You have reached the end of Physics lesson 19.2.2 Einstein's Equation of Photoelectric Effect. The Particle Nature of Light. There are 3 lessons in this physics tutorial covering The Photoelectric Effect, you can access all the lessons from this tutorial below.
More The Photoelectric Effect Lessons and Learning Resources
Whats next?
Enjoy the "Einstein's Equation of Photoelectric Effect. The Particle Nature of Light" physics lesson? People who liked the "The Photoelectric Effect lesson found the following resources useful:
- Einsteins Equation Feedback. Helps other - Leave a rating for this einsteins equation (see below)
- Modern Physics Physics tutorial: The Photoelectric Effect. Read the The Photoelectric Effect physics tutorial and build your physics knowledge of Modern Physics
- Modern Physics Revision Notes: The Photoelectric Effect. Print the notes so you can revise the key points covered in the physics tutorial for The Photoelectric Effect
- Modern Physics Practice Questions: The Photoelectric Effect. Test and improve your knowledge of The Photoelectric Effect with example questins and answers
- Check your calculations for Modern Physics questions with our excellent Modern Physics calculators which contain full equations and calculations clearly displayed line by line. See the Modern Physics Calculators by iCalculator™ below.
- Continuing learning modern physics - read our next physics tutorial: The Compton Effect and Pressure of Light
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "The Photoelectric Effect" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Modern Physics Calculators by iCalculator™
- Characteristic Em Wavelength Calculator
- De Broglie Wave Calculator
- Rayleigh Jeans Relation Calculator
- Energy Of Photons Calculator
- Intensity Photoelectric Effect Calculator
- Kinetic Photoelectric Effect Calculator
- Light Pressure Calculator
- Radiation Black Body Calculator
- Stopping Voltage Photoelectric Effect Calculator
- Uncertainty Calculator
- Wave Width Calculator
- Scattered Radiation Compton Effect Calculator
- De Broglie Packet Calculator