Menu
Physics Lesson 9.4.4 - Gas Pressure
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Gas Pressure, this is the fourth lesson of our suite of physics lessons covering the topic of Gas Pressure, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Gas Pressure
We cannot use standard barometers to measure pressure of a specific gas, as we would need a closed environment in which there is only this gas. Therefore, we use a combination of U-shaped tube and barometer as an equipment to measure gas pressure if the value of atmospheric pressure is known. This equipment is called "open-tube manometer" or "pressure gauge" which consists in a closed deposit at one side, in which there is the gas whose pressure needs to be measured, and an open end at the other side of a U-shaped tube, which is in contact with the air (atmospheric pressure). The separation between these two gases (air and the specific gas to be measured) is ensured by a liquid, such as water, mercury etc., as shown in the figure.
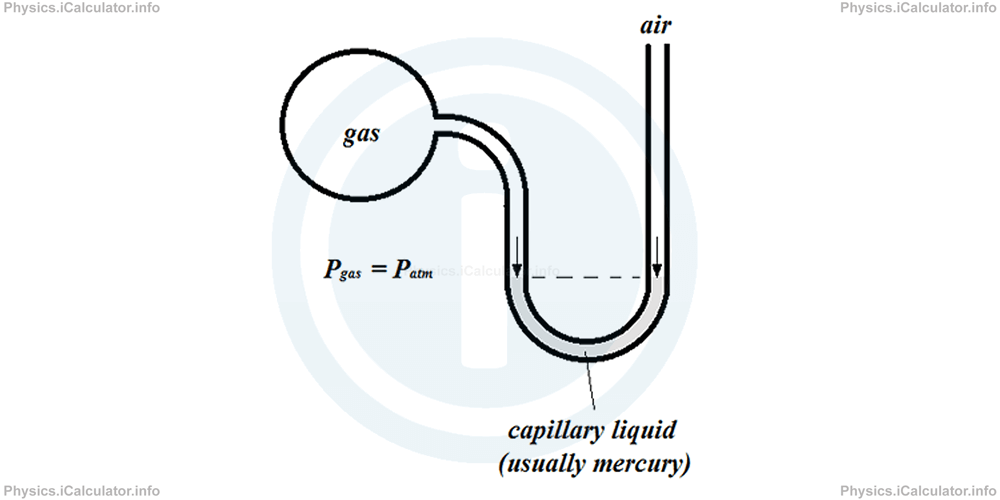
1) In the case shown above, air pressure is equal to the gas pressure as both gases push equally the capillary liquid at bottom of the U-shaped tube. As a result, the liquid level is the same in both sides of the tube.
If the capillary liquid inside the manometer is mercury, we can write
2) When gas pressure is greater than atmospheric pressure, the gas pushes the capillary liquid more than air. As a result, there will be a disparity in the liquid level in both sides of the U-shaped tube, as shown below.
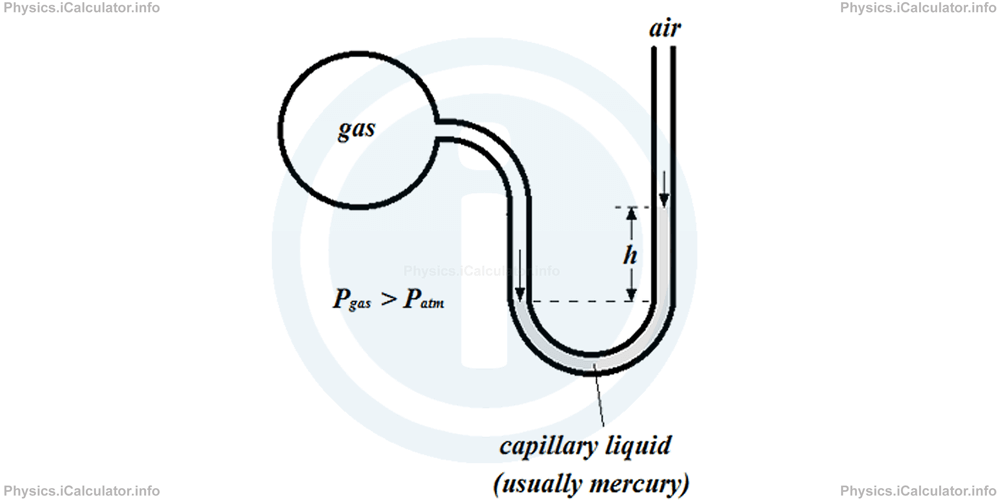
The abovementioned disparity is represented mathematically by the height h, similarly as in the U-shaped tube used to calculate the density of an unknown liquid discussed in the Physics tutorial Physics Tutorial: Liquid Pressure. Pascal's Principle
Mathematically we have:
3) When air pressure is greater than gas pressure, the air will push the capillary liquid more than the gas. As a result, we will obtain the following figure.
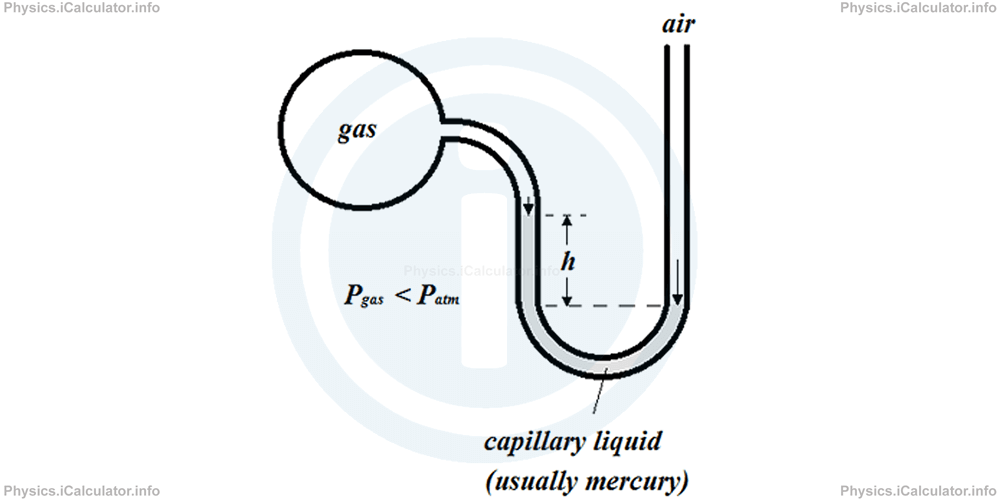
Mathematically we have:
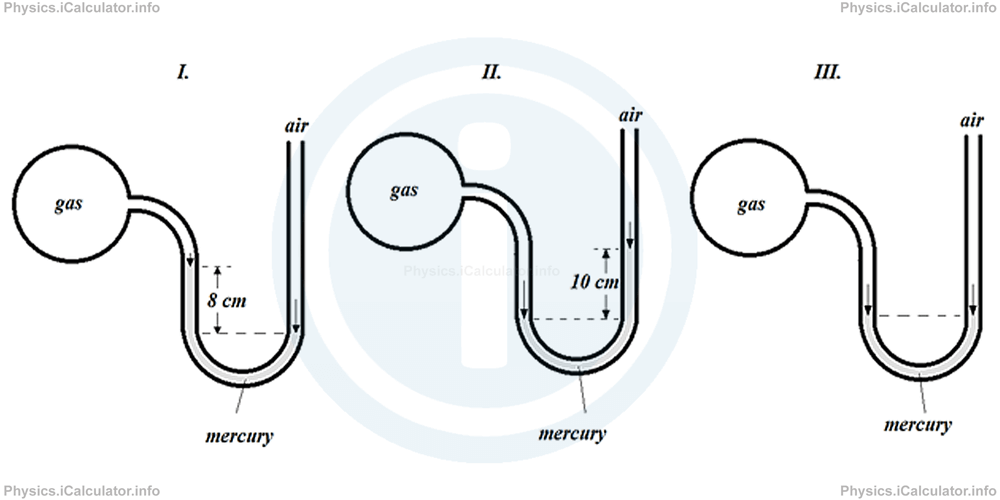
Example 3
Calculate the gas pressure (in cm - Hg) in the three examples shown below if the actual atmospheric pressure is 74 cm - Hg.
Solution 3
I. In this case, Patm > Pgas and h = 8 cm - Hg. Thus, given that Patm = 74 cm - Hg, we obtain for the gas pressure:
= 74 cm - Hg - 8 cm - Hg
= 66 cm - Hg
II. In this case, Patm < Pgas and h = 10 cm - Hg. Thus, given that Patm = 74 cm - Hg, we obtain for the gas pressure:
= 74 cm - Hg + 10 cm - Hg
= 84 cm - Hg
III. In this case, Patm = Pgas as mercury level is the same in both sides of the tube. Therefore, since Patm = 74 cm - Hg, we obtain the same value for the gas pressure, i.e.
Remark! The atmospheric pressure in normal conditions is often referred as P0 instead of Patm.
You have reached the end of Physics lesson 9.4.4 Gas Pressure. There are 6 lessons in this physics tutorial covering Gas Pressure, you can access all the lessons from this tutorial below.
More Gas Pressure Lessons and Learning Resources
Whats next?
Enjoy the "Gas Pressure" physics lesson? People who liked the "Gas Pressure lesson found the following resources useful:
- Definition Feedback. Helps other - Leave a rating for this definition (see below)
- Density and Pressure Physics tutorial: Gas Pressure. Read the Gas Pressure physics tutorial and build your physics knowledge of Density and Pressure
- Density and Pressure Revision Notes: Gas Pressure. Print the notes so you can revise the key points covered in the physics tutorial for Gas Pressure
- Density and Pressure Practice Questions: Gas Pressure. Test and improve your knowledge of Gas Pressure with example questins and answers
- Check your calculations for Density and Pressure questions with our excellent Density and Pressure calculators which contain full equations and calculations clearly displayed line by line. See the Density and Pressure Calculators by iCalculator™ below.
- Continuing learning density and pressure - read our next physics tutorial: Buoyancy. Archimedes' Principle
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Gas Pressure" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.