Menu
Physics Lesson 9.4.2 - Air (Atmospheric) Pressure
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Air (Atmospheric) Pressure, this is the second lesson of our suite of physics lessons covering the topic of Gas Pressure, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Air (Atmospheric) Pressure
The first scientist who was successful in his attempts to measure the air pressure was Evangelista Torricelli, an Italian physicist and mathematician and a student of Galileo Galilei. He invented an air pressure gauge called "mercury barometer" or simply "barometer" which consists of an open vessel with a closed vertical tube at it middle as shown in the figure.
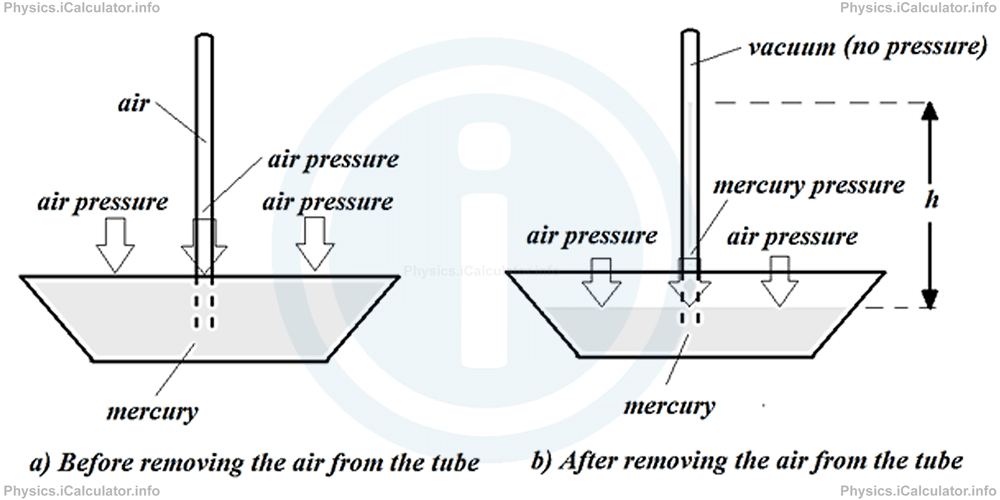
When the vessel is filled with mercury, its level is the same everywhere, even inside the tube (figure a), as this setup is a communicating vessel, as discussed in the previous tutorial "Liquid Pressure. Pascal's Principle ." This is because air exerts the same pressure on all parts of mercury surface.
If we remove the air from the upper part of the tube, mercury will start to raise up inside the tube, as there is no air above to hamper it from raising up. In this case, mercury acts as a capillary liquid, i.e. as a liquid that rises up in narrow tubes because of the change in pressure. This raise will continue until the liquid pressure caused by the mercury column will balance the air pressure aside (figure b). Therefore, since it is easy to measure the height h of mercury column and given that density of mercury is known (it is 13 600 kg/m3 or 13.6 g/cm3), we can calculate the air (atmospheric) pressure by working out the pressure of mercury column, i.e.
= ρmercury × g × hmercury column
In normal atmospheric conditions (at sea level, in a sunny day at 150C), the mercury inside the column rises at 760 mm (76.0 cm or 0.760 m) above the neighbouring parts in contact with air. As a result, the atmospheric pressure in normal conditions is
≈ 101 325 Pa
Thus, we can write:
(Hg is the chemical symbol of mercury)
The above value can also be written as 1 atm (atmosphere). Thus, we have:
To facilitate calculations, another unit known as "bar" is often used. The conversion factor between bar and Pa is
Thus, we can say bar is slightly smaller than atm, as
On the other hand,
= 1.01325 bar
= 1013.25 mb
(mb means millibar)
High atmospheric pressure means the barometer shows values greater than 76 cm - Hg. Such a condition corresponds to cloudy skies and precipitations, as air charged with moisture is concentrated in the lower part of the sky. This increases the value of air pressure because an extra factor (moisture, i.e. water droplets) adds to the normal air pressure, giving a higher value in the barometer. On the other hand, low atmospheric pressure is associated with clear skies because the level of moisture in the air is very low.
Example 1
The barometer in a weather station shows 74 cm - Hg.
- What kind of weather is at the weather station location?
- What is the value of atmospheric pressure in Pa and millibar (mb)?
Solution 1
- The value shown by the barometer (74 cm - Hg) is smaller than the value of normal atmospheric pressure (76 cm - Hg). This means there is a good weather (clear sky) in the given location.
- We use the cross-production rule to convert the value in the desired units. Thus,
74 cm - Hg = x Pa
We have
= 98 659 Pa
The same procedure is used to convert the atmospheric pressure from Pa to millibar. Thus,
98 659 Pa = x bar
We have
= 986.59 mb
You have reached the end of Physics lesson 9.4.2 Air (Atmospheric) Pressure. There are 6 lessons in this physics tutorial covering Gas Pressure, you can access all the lessons from this tutorial below.
More Gas Pressure Lessons and Learning Resources
Whats next?
Enjoy the "Air (Atmospheric) Pressure" physics lesson? People who liked the "Gas Pressure lesson found the following resources useful:
- Atmospheric Feedback. Helps other - Leave a rating for this atmospheric (see below)
- Density and Pressure Physics tutorial: Gas Pressure. Read the Gas Pressure physics tutorial and build your physics knowledge of Density and Pressure
- Density and Pressure Revision Notes: Gas Pressure. Print the notes so you can revise the key points covered in the physics tutorial for Gas Pressure
- Density and Pressure Practice Questions: Gas Pressure. Test and improve your knowledge of Gas Pressure with example questins and answers
- Check your calculations for Density and Pressure questions with our excellent Density and Pressure calculators which contain full equations and calculations clearly displayed line by line. See the Density and Pressure Calculators by iCalculator™ below.
- Continuing learning density and pressure - read our next physics tutorial: Buoyancy. Archimedes' Principle
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Gas Pressure" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.