Menu
Physics Lesson 9.4.6 - How does the Atmospheric Pressure Vary with Altitude?
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on How does the Atmospheric Pressure Vary with Altitude?, this is the sixth lesson of our suite of physics lessons covering the topic of Gas Pressure, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
How does the Atmospheric Pressure Vary with Altitude?
The pressure at any level in the atmosphere represents the total weight of the air above a unit area. At higher altitudes, there are fewer air molecules above a given surface than on a similar surface at lower levels. For example, there are fewer molecules at 50 km above the Earth surface than at 10 km of altitude.
The following figure shows the air pressure values in some altitudes.
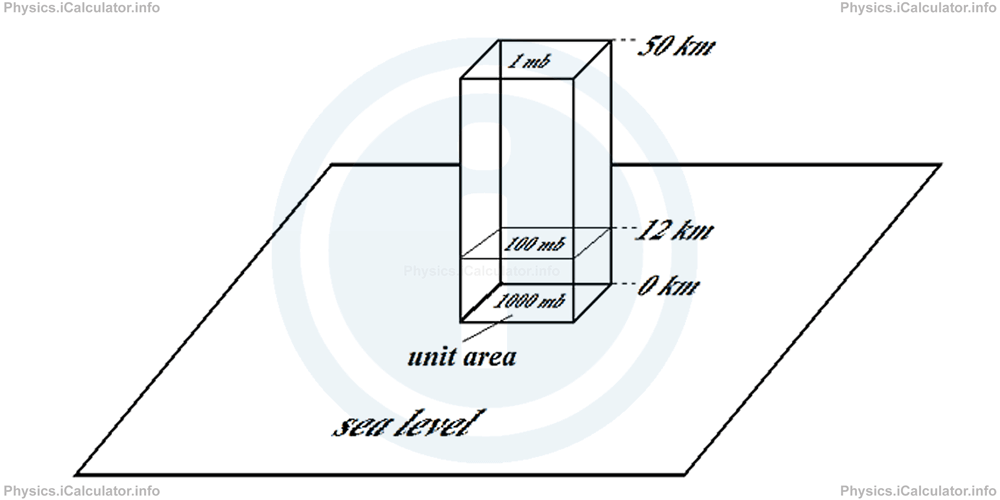
Atmospheric pressure decreases with the increase in altitude. Since most of the atmosphere's molecules are near the earth's surface because of the attracting force of gravity, air pressure decreases rapidly at first (from sea level to h = 12 km), then more slowly at higher altitudes (from h = 12 km to h = 50 km) as air becomes very rare. Thus, since more than half of air molecules are in the first 5.5 km of atmosphere, air pressure halves at that altitude, i.e. Pair (5.5 km) = 500 mb = 1/2 P0. (This altitude is not shown in the figure in order to not make it confusing.) Such a decrease continues, albeit at slower rate, until it becomes 1 mb or 1/1000 P0 at h = 50 km.
There is a mathematical formula, which expresses the relationship between air pressure and altitude. Below is shown a simplified version of it.
where P0 = 101 325 Pa is the standard atmospheric pressure in normal conditions and h is the altitude in metres. The other numbers are values obtained from operations with various constants.
Example 5
What is the value of air pressure in the boundaries of ozone layer? Most of ozone lies from 10 km to 16 km above the sea level. Take the atmospheric conditions at sea level as normal (standard).
Solution 5
Let's write the values of altitude and air pressure at sea level in standard notation. We have h1 = 10 km = 104 m and h2 = 16 km = 1.6 × 104 m. Also, P0 = 101 325 Pa = 1.01325 × 105 Pa.
Since
we obtain for P1 (P at 10 km above sea level):
= 1.01325 × 105 × (1 - 2.25577 × 10-1 )5.25588
= 1.01325 × 105 × (1 - 0.225577)5.25588
= 1.01325 × 105 × (0.774423)5.25588
= 1.01325 × 105 × 0.2609
= 0.26436 × 105 Pa
= 26 436 Pa
and for P2 (at 16 km above the sea level):
= 1.01325 × 105 × (1 - 3.609232 × 10-1 )5.25588
= 1.01325 × 105 × 0.6395.25588
= 1.01325 × 105 × 0.095
= 0.09626 × 105 Pa
= 9626 Pa
As you see, these values are very small when compared to standard atmospheric pressure. This means the air density is very low in such altitudes as air pressure is produced when air molecules hit a given surface by a certain force, i.e. it is proportional to the number of air molecules available.
You have reached the end of Physics lesson 9.4.6 How does the Atmospheric Pressure Vary with Altitude?. There are 6 lessons in this physics tutorial covering Gas Pressure, you can access all the lessons from this tutorial below.
More Gas Pressure Lessons and Learning Resources
Whats next?
Enjoy the "How does the Atmospheric Pressure Vary with Altitude?" physics lesson? People who liked the "Gas Pressure lesson found the following resources useful:
- Altitude Feedback. Helps other - Leave a rating for this altitude (see below)
- Density and Pressure Physics tutorial: Gas Pressure. Read the Gas Pressure physics tutorial and build your physics knowledge of Density and Pressure
- Density and Pressure Revision Notes: Gas Pressure. Print the notes so you can revise the key points covered in the physics tutorial for Gas Pressure
- Density and Pressure Practice Questions: Gas Pressure. Test and improve your knowledge of Gas Pressure with example questins and answers
- Check your calculations for Density and Pressure questions with our excellent Density and Pressure calculators which contain full equations and calculations clearly displayed line by line. See the Density and Pressure Calculators by iCalculator™ below.
- Continuing learning density and pressure - read our next physics tutorial: Buoyancy. Archimedes' Principle
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Gas Pressure" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.