Menu
Physics Lesson 13.1.3 - Temperature and Thermal Equilibrium. The Zeroth Law of Thermodynamics
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Temperature and Thermal Equilibrium. The Zeroth Law of Thermodynamics, this is the third lesson of our suite of physics lessons covering the topic of Temperature. The Zeroth Law of Thermodynamics, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Temperature and Thermal Equilibrium. The Zeroth Law of Thermodynamics
As written in the definition, temperature represents a measure of the ability of an object or a system of objects to transfer heat energy to another object or system. But before explaining what this definition really means, it is necessary to explain a few concepts, such as heat energy, thermal energy, internal energy and heat transfer.
Objects possess energy in a variety of forms. Some types of energy are easily visible and measurable such as kinetic energy, gravitational potential energy and elastic potential energy we have explained earlier in Section 5. However, there are some other types of energy the objects possess in a microscopic form, which are not easy to be identified and calculated. These energies belong to the category of internal energy. The two main subcategories of internal energy are chemical and thermal energy. The first involves the energy generation or absorption during chemical reactions. The later involves the energy generation during the local movements (like vibrations) of objects' particles due to their hotness, as explained earlier. Therefore, it is easy to conclude that thermal energy is somehow related to temperature, i.e. hot objects possess more thermal energy than cold objects as their particles vibrate more rapidly.
The part of this thermal energy that is transferred to another object, is known as heat energy. As explained in the Physics tutorial 5.1 "Work and Energy. Types of Energy", heat can be transferred from one object into another when the proper conditions are created. One condition would be putting a hot and a cold object in contact to each other and thus, paving the way to the heat energy to transfer from the hottest object to the coldest one. There is not any matter transfer during this process; the only thing that is transferred is the heat energy due to the collision pf particles in the bordering parts between the two objects. Faster particles in the outer layer of the hot object collide with the slower particles of the outer layer of the cold object. In this way, the hot object loses some heat energy as its particles get slower during the collision and the cold object gains some heat energy because its particles become faster during the collision with more energetic particles. As a result, there is a heat exchange between objects which eventually bring a change in the internal energy of both objects.
The heat "flow" eventually stops when both objects have reached the same temperature. This means the average speeds of particles vibration in both objects are already equal.
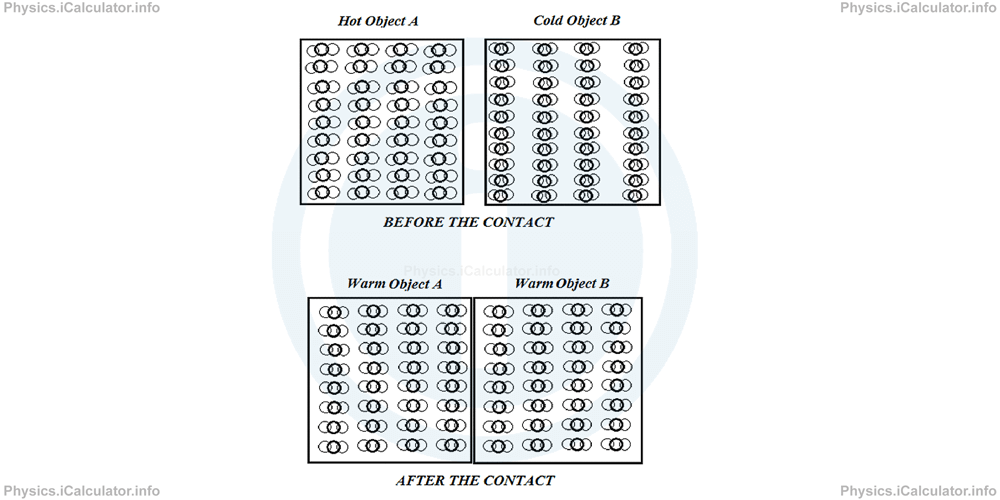
In other words, the heat flow stops when thermal equilibrium is established. In this way, we obtained the meaning of thermal equilibrium, i.e. a condition in which all parts of a system are at the same temperature.
It is precisely on this concept that the Zeroth Law of Thermodynamics is based. It states that:
If a thermodynamic system A is in thermal equilibrium with another thermodynamic system B and the thermodynamic system B is in thermal equilibrium with a third thermodynamic system C, then the thermodynamic system A is also in thermal equilibrium with the thermodynamic system C.
A thermodynamic system is an object of a group of objects with the same temperature. For simplicity, we will consider the system as a single object. Thus, in simpler words, the Zeroth Law of Thermodynamics says:
If an object A has the same temperature with another object B and the object B has the same temperature with a third object C, then the object A has the same temperature with the object C.
For example, the object in the factory in which a thermometer was originally placed in contact to do its calibration represents the system A, the thermometer itself is the system B and the patient's body is the system C.
The Zeroth Law of Thermodynamics is schematically shown below.
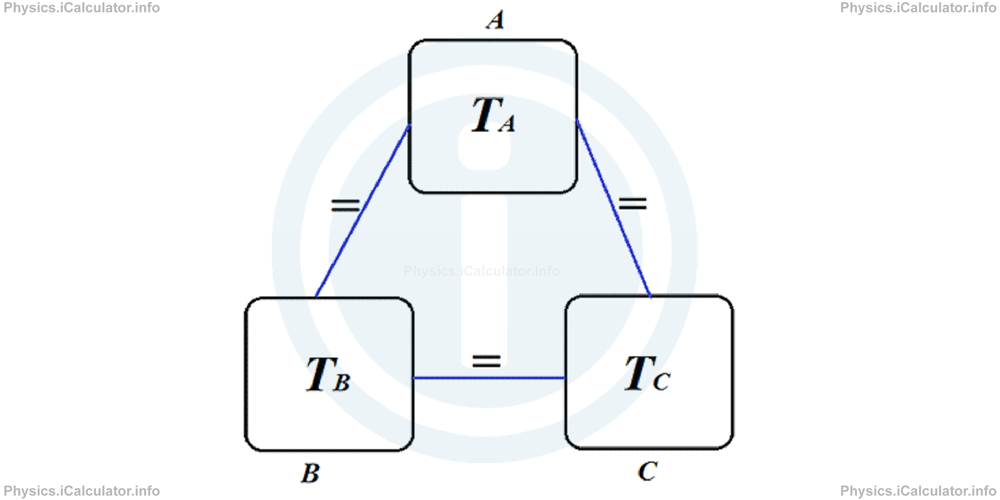
It is called the "Zeroth Law of Thermodynamics" as it was formulated after the First and the Second Laws of Thermodynamics, for which we will discuss in the next tutorials.
You have reached the end of Physics lesson 13.1.3 Temperature and Thermal Equilibrium. The Zeroth Law of Thermodynamics. There are 3 lessons in this physics tutorial covering Temperature. The Zeroth Law of Thermodynamics, you can access all the lessons from this tutorial below.
More Temperature. The Zeroth Law of Thermodynamics Lessons and Learning Resources
Whats next?
Enjoy the "Temperature and Thermal Equilibrium. The Zeroth Law of Thermodynamics" physics lesson? People who liked the "Temperature. The Zeroth Law of Thermodynamics lesson found the following resources useful:
- Equilibrium Feedback. Helps other - Leave a rating for this equilibrium (see below)
- Thermodynamics Physics tutorial: Temperature. The Zeroth Law of Thermodynamics. Read the Temperature. The Zeroth Law of Thermodynamics physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Temperature. The Zeroth Law of Thermodynamics. Print the notes so you can revise the key points covered in the physics tutorial for Temperature. The Zeroth Law of Thermodynamics
- Thermodynamics Practice Questions: Temperature. The Zeroth Law of Thermodynamics. Test and improve your knowledge of Temperature. The Zeroth Law of Thermodynamics with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Thermal Expansion
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Temperature. The Zeroth Law of Thermodynamics" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator