Menu
Physics Lesson 13.7.1 - Relationship between Pressure, Temperature and RMS Speed in a Gas
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Relationship between Pressure, Temperature and RMS Speed in a Gas, this is the first lesson of our suite of physics lessons covering the topic of Pressure, Temperature and RMS Speed, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Relationship between Pressure, Temperature and RMS Speed in a Gas
Let's suppose we have n moles of a gas enclosed within a cubic container of volume V and side length L, as shown in the figure below.
The container walls are held at constant temperature T. Let's find the relationship between the gas pressure exerted at the walls and the speed of molecules.
Molecules move in every direction within the container; they hit each other and the container walls and then, they bounce back. If we consider the gas as ideal, the collisions between particles can be neglected. Thus, we consider only the elastic collisions of gas molecules with the container walls.
When a molecule of mass m collides normally with the walls of the container at velocity v, it turns back at velocity -v as shown in the figure.
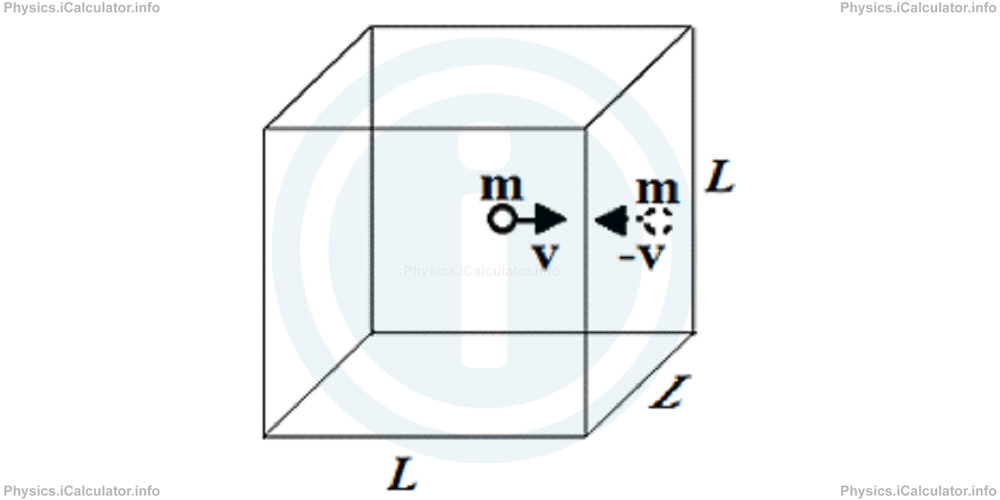
This means the change in momentum Δp of the molecule is
= m × ∆v
= m × v - m × (-v)
= 2 × m × v
This process is repeated continuously between the two opposite walls. Given that the distance between the container walls is L, the time interval Δt needed for the molecule to turn again to the original position is
From the Newton's Second Law of Motion, we have for the force by which the molecule hits the container wall:
= m × ∆v/∆t
= m × ∆v/∆t
= ∆p/∆t
Substituting the values obtained earlier, we get:
= 2 × m × v/2L/v
= m × v2/L
Given that Pressure = Force / Area, we obtain for the pressure exerted by the molecule on the container wall:
= m × v2/L/L2
= m × v2/L3
For a large number of molecules, we can write
where N is the total number of molecules in the container.
Thus, given that N = n × NA and (v21 + v22 + ... + v2N ) = v2average, we obtain
We have m × NA = M and L3 = V, where M is the molar mass of gas and V is the volume of container. Thus, we obtain
We have considered only one direction so far (let's say only the x-direction. If we consider all three directions available for the motion of molecules, we obtain:
Therefore, the equation of gas pressure for a single direction becomes
We call the quantity √v2average as root mean square speed (vrms). Hence, we can write
The last formula indicates how a macroscopic quantity such as pressure depends on a microscopic quantity such as the speed of molecules.
From the ideal gas law, we know that
Therefore, combining the last two formulae, we obtain
R × T = M/3 × v2rms
v2rms = 3 × R × T/M
vrms = √3 × R × T/M
Example 1
What is the rms speed of CO2 molecules at 27°C if their molar mass is 44 g/mol?
Solution 1
Clues:
M = 44 g/mol = 0.44 kg/mol
T = 27°C = 300 K
R = 8.31 J/molK
vrms = ?
Using the equation
we obtain after substitutions
This is a very big value; it results the CO2 molecules move at the speed of bullets when they come out of the riffle muzzle. However, the real rms speed is much smaller as besides the collision with the container walls molecules collide with each other as well - a speed which we neglected when considering the gas as ideal. Also, the value found is an average value; there are many molecules which move slower.
You have reached the end of Physics lesson 13.7.1 Relationship between Pressure, Temperature and RMS Speed in a Gas. There are 3 lessons in this physics tutorial covering Pressure, Temperature and RMS Speed, you can access all the lessons from this tutorial below.
More Pressure, Temperature and RMS Speed Lessons and Learning Resources
Whats next?
Enjoy the "Relationship between Pressure, Temperature and RMS Speed in a Gas" physics lesson? People who liked the "Pressure, Temperature and RMS Speed lesson found the following resources useful:
- Relationships Feedback. Helps other - Leave a rating for this relationships (see below)
- Thermodynamics Physics tutorial: Pressure, Temperature and RMS Speed. Read the Pressure, Temperature and RMS Speed physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Pressure, Temperature and RMS Speed. Print the notes so you can revise the key points covered in the physics tutorial for Pressure, Temperature and RMS Speed
- Thermodynamics Practice Questions: Pressure, Temperature and RMS Speed. Test and improve your knowledge of Pressure, Temperature and RMS Speed with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Molar Specific Heats and Degrees of Freedom
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Pressure, Temperature and RMS Speed" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator