Menu
Physics Lesson 13.7.3 - Mean Free Path
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Mean Free Path, this is the third lesson of our suite of physics lessons covering the topic of Pressure, Temperature and RMS Speed, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Mean Free Path
If you throw a few potato starch on water surface, you will notice that starch grains move in random direction. Furthermore, grain particles change direction continuously as shown in the figure.
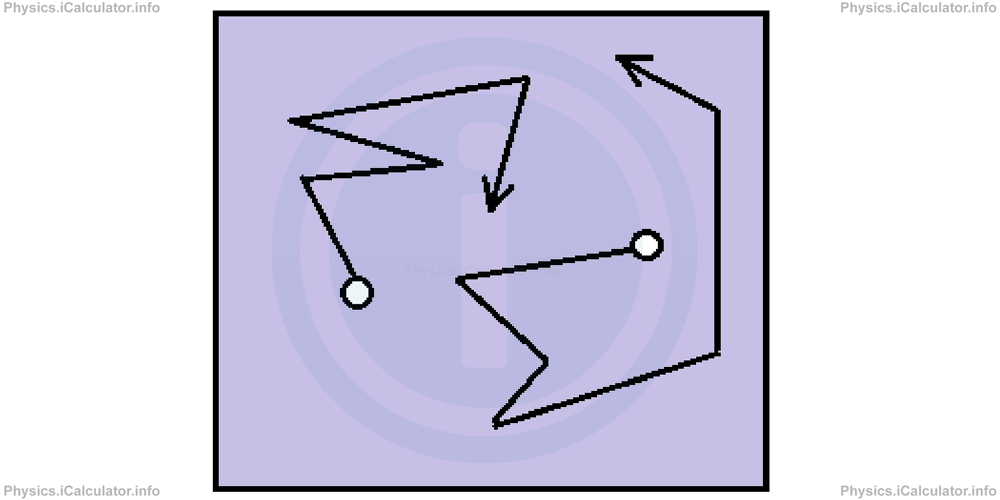
This phenomenon occurs because starch particles are hit by water molecules, which we cannot see as they are very small.
The same phenomenon occurs in gas particles as well. They collide continuously with each other; however, they move linearly at constant speed during the time interval between two consecutive collisions.
We use a parameter known as "mean free path" (in symbols, λ) to describe this random motion of gas particles. It represents the average distance travelled by a gas particle between two collisions. Mean free path depends on the following factors:
1- Density or concentration of gas. In this case, we express density in terms of concentration of molecules, i.e. as number of molecules per unit volume (in short, N/V) instead of mass per unit volume, as here we are interested on microscopic parameters such as the number of molecules.
Based on this factor, we can say that higher the concentration of particles, smaller their mean free path. Therefore, the molecular mean free path is inversely proportional to gas concentration.
2- Size of molecules. Obviously, larger the gas molecules, shorter the mean free path between them. molecules are, the smaller the mean free path. Mean free path λ, should vary (inversely) as the square of the molecular diameter because the cross section of a molecule - not its diameter - determines its effective target area.
Let's find the correct expression for the mean free path. Consider two spherical molecules of diameter d. If we insert them in the opposite ends of a cylindrical tube, the molecules will collide with each other only if the diameter of cylinder is smaller than or equal to 2d as shown in the figure below.
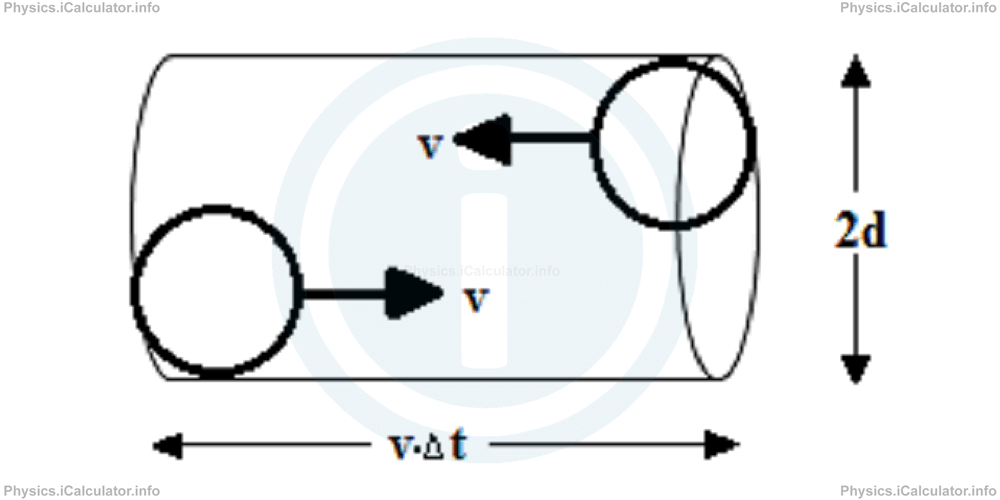
A single molecule moving at speed v travels the distance v × Δt between two collisions. This corresponds to the height of the cylinder discussed earlier. Therefore the volume of cylinder is
Therefore, mean free path is
= v × ∆t/N
= v × ∆t/V × N/V
= v × ∆t/π × d2 × v × ∆t × N/V
= 1/π × d2 × N/V
The above equation does assumes all the other molecules at rest except one. This is obviously not true. If we want to take a more accurate result, we must consider a correction factor 1/√2. Therefore, we obtain for the mean free path of gas molecules:
Example 3
: 2 moles of a gas are enclosed inside a cubic container of side length equal to 4 m. The molecules of gas have a diameter of 10-10 m each. What is the mean free path of gas molecules?Solution 3
We have a = 4 m, so V = a3 = (4 m)3 = 64 m3.
Also, N = n × NA = 2 moles × 6.02 × 1023 molecules/mole = 12.04 × 1023 molecules.
Therefore, we obtain for the mean free path λ:
= 1/√2 × 3.14 × (10-10)2 × 12.04 × 1023/64
= N/0.835 × 103
= 1.198 × 10-3 m
= 1.198 mm
You have reached the end of Physics lesson 13.7.3 Mean Free Path. There are 3 lessons in this physics tutorial covering Pressure, Temperature and RMS Speed, you can access all the lessons from this tutorial below.
More Pressure, Temperature and RMS Speed Lessons and Learning Resources
Whats next?
Enjoy the "Mean Free Path" physics lesson? People who liked the "Pressure, Temperature and RMS Speed lesson found the following resources useful:
- Mean Free Path Feedback. Helps other - Leave a rating for this mean free path (see below)
- Thermodynamics Physics tutorial: Pressure, Temperature and RMS Speed. Read the Pressure, Temperature and RMS Speed physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Pressure, Temperature and RMS Speed. Print the notes so you can revise the key points covered in the physics tutorial for Pressure, Temperature and RMS Speed
- Thermodynamics Practice Questions: Pressure, Temperature and RMS Speed. Test and improve your knowledge of Pressure, Temperature and RMS Speed with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Molar Specific Heats and Degrees of Freedom
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Pressure, Temperature and RMS Speed" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator