Menu
Physics Lesson 13.9.1 - Relationship between Pressure and Volume at Constant Temperature. The Boyle's Law
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Relationship between Pressure and Volume at Constant Temperature. The Boyle's Law, this is the first lesson of our suite of physics lessons covering the topic of Gas Laws, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Relationship between Pressure and Volume at Constant Temperature. The Boyle's Law
Let's consider again the virtual experiment of the previous tutorials with the gas sample enclosed inside the cylinder with a freely moveable piston on top of the gas. We discussed this situation in the previous tutorial when explaining the work done by the gas to move the piston upwards by Δh, which causes a change in gas volume by ΔV.
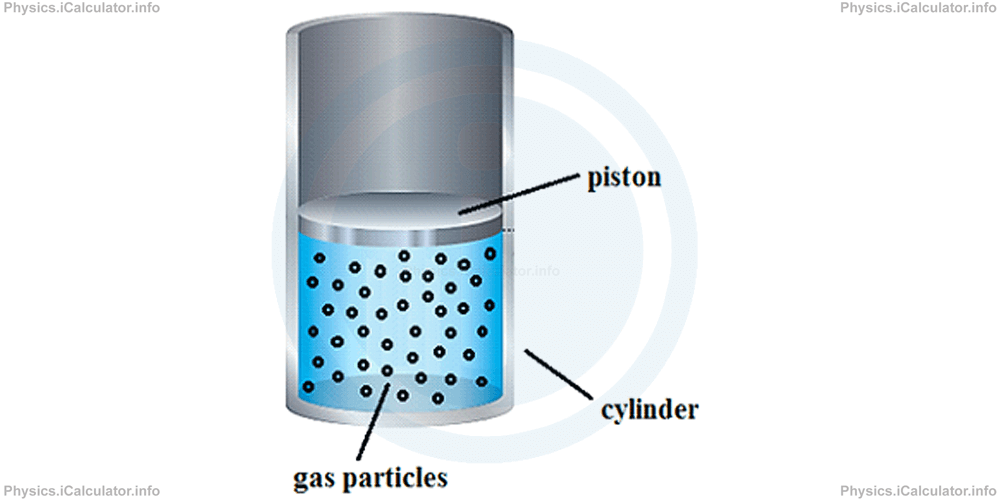
Let's suppose the gas is initially in the state shown above. We have call it the "initial state" of the gas. The gas parameters for this state are denoted as P1, V1 and T1 where P stands for pressure, V for volume and T for temperature.
When we gently press the piston from up, it will lower down causing a compression of the gas. As a result, the gas volume decreases until a new equilibrium is settled. This brings an increase in the gas pressure as the same number of particles collide with a smaller area (the internal area of cylinder walls), given that
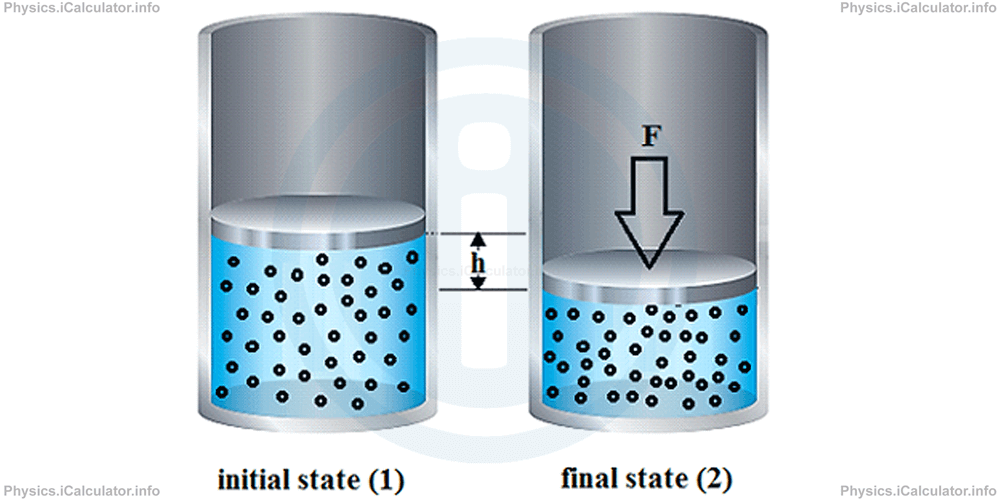
If we assume the gas as ideal, i.e. no collision between particles does occur; only collisions between particles and cylinder walls are considered, the gas will have some new parameters when the new equilibrium is established. We write them as P2, V2 and T2.
Since in this case we simply push the piston gently down, no change in temperature occurs in gas. Hence, we can write just T for both states instead of T1 and T2. Such a process in which the temperature remains constant during the entire event is known as isothermal process ("iso" = constant, while the term "thermal" implies the temperature. Thus, isothermal process means a process with constant temperature).
If we write the ideal gas law for the two above states, we obtain:
for the initial state and
for the final state of the gas, where n is the number of moles and R is the ideal gas constant (both remain unchanged during the entire process).
When dividing the above equations with each other, we obtain
Rearranging the last formula, we obtain the relationship between pressure and volume at constant temperature. It is known as the Boyle's Law and mathematically, it is written as
Example 1
A balloon containing 2 L of air is pressed as shown in the figure.

As a result, the air inside the balloon is compressed to a volume of 1.25 L. What is the new air pressure inside the balloon (in atm) if this process occurs at standard atmospheric pressure? Assume the air inside the balloon as an ideal gas.
Solution 1
Since the process occurs without any change in temperature (no heat sources involved), we can apply the Boyle's Law for the two states: initial (1) and final (2) instead of the ideal gas law. Given that the standard atmospheric pressure is 1 atm, we can write the following quantities as clues:
V1 = 2 L
V2 = 1.25 L
T1 = T2 = T
P1 = 1 atm
P2 = ?
From Boyle's Law, we have
Substituting the values, we obtain for the final pressure of air inside the balloon:
P2 = 1 atm × 2 L/1.25 L
= 1.6 atm
The Pressure vs Volume graph for an isothermal process is shown below:
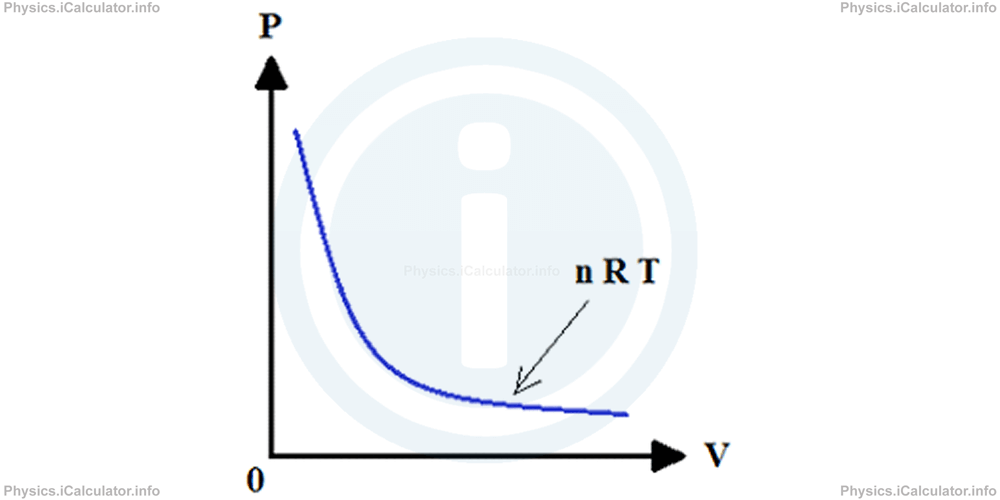
This graph if a hyperbola (in math, a hyperbola is the graph of functions of the form y = k / x. It is also known as the inverse variation function, in which when the independent variable x increases by a factor of k, the dependent variable y decreases by the same factor).
In Boyle's Law, the constant k is represented by the expression n × R × T as at constant temperature T we obtain by rearranging the ideal gas formula:
or
Example 2
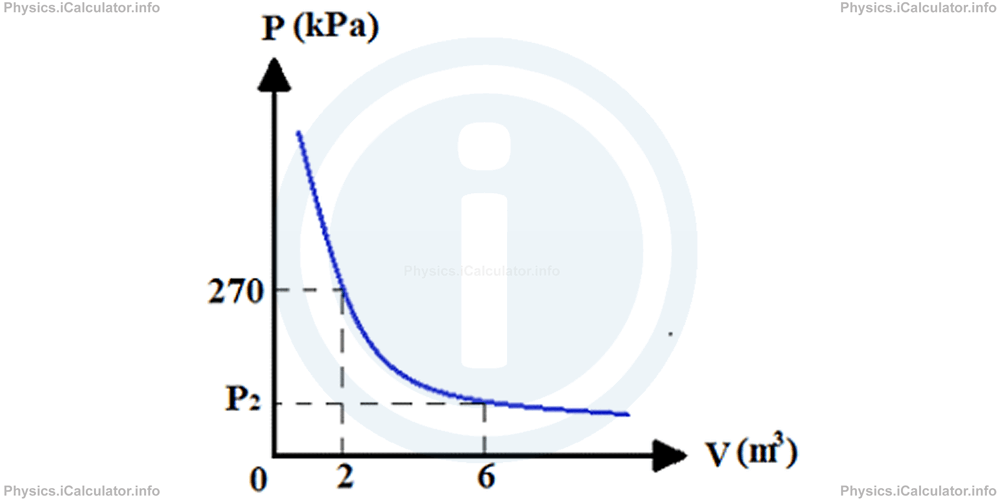
Determine the pressure and temperature of an ideal gas sample, whose P - V graph is shown below given that 1 mole of an ideal gas at standard atmospheric pressure and normal temperature occupies 22.4 L of space.
Solution 2
First, we find the final pressure P2 applying the Boyle's Law given that the graph represents an isothermal process. We have P1 = 270 kPa, V1 = 2 L, V2 = 6 L. Thus,
P2 = P1 × V1/V2
= 270 kPa × 2 L/6
= 90 kPa
As for gas temperature, it can be calculated by considering the coordinates of any known point of the graph, as the temperature is constant during the entire process. But first, we have to calculate the number of gas moles in standard atmospheric pressure. We can take either the initial or the final parameters of gas (let's say the initial ones) and the value of standard atmospheric pressure and use again the Boyle's Law to determine the normal volume of gas. Thus, we have
100 kPa × V0 = 270 kPa × 2 L
V0 = 270 kPa × 2 L/100 kPa = 5.4 L
Hence, the number of moles n is
= 5.4 L/22.4 L/mol
= 0.241 moles
Thus, taking the values of the initial state P1 = 270 kPa = 270000 Pa and V1 = 2 L = 0.002 m3, we obtain for temperature by rearranging the ideal gas law for this state:
= 270000 Pa × 0.002 m3/0.241 moles × 8.31 J/mol × K
= 540 J/2 J/K
= 270 K
Remark!
It is scientifically correct to convert all values of pressure, volume and temperature in the standard SI units. However, it is OK even if you use other units for pressure and volume when applying the Boyle's Law if the same unit is used in both states. Only temperature must be definitely converted into Kelvin degrees as gas laws work only when temperature is expressed in Kelvin scale.
You have reached the end of Physics lesson 13.9.1 Relationship between Pressure and Volume at Constant Temperature. The Boyle's Law. There are 4 lessons in this physics tutorial covering Gas Laws, you can access all the lessons from this tutorial below.
More Gas Laws Lessons and Learning Resources
Whats next?
Enjoy the "Relationship between Pressure and Volume at Constant Temperature. The Boyle's Law" physics lesson? People who liked the "Gas Laws lesson found the following resources useful:
- Boyle Feedback. Helps other - Leave a rating for this boyle (see below)
- Thermodynamics Physics tutorial: Gas Laws. Read the Gas Laws physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Gas Laws. Print the notes so you can revise the key points covered in the physics tutorial for Gas Laws
- Thermodynamics Practice Questions: Gas Laws. Test and improve your knowledge of Gas Laws with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Entropy and the Second Law of Thermodynamics
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Gas Laws" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator