Menu
Physics Lesson 13.5.6 - Special Cases of the First Law of Thermodynamics
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Special Cases of the First Law of Thermodynamics, this is the sixth lesson of our suite of physics lessons covering the topic of The First Law of Thermodynamics, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Special Cases of the First Law of Thermodynamics
There are four special cases in the application of the First Law of Thermodynamics.
I. Adiabatic process.
This process occurs very rapidly or it occurs in such a well-insulated system, so that no heat is transferred to or by the system. This means Q = 0 and the mathematical expression of the First Law of Thermodynamics becomes
0 = ∆U + Wby the system
∆U = -Wby the system
This means all the work done by an external force to the system goes for the increase in its internal energy.
Example 2
If we push down very fast a 200 g piston by 15 cm, what is the final internal energy of the gas if initially it had 1.4 J of internal energy? Take g = 10 N/kg.
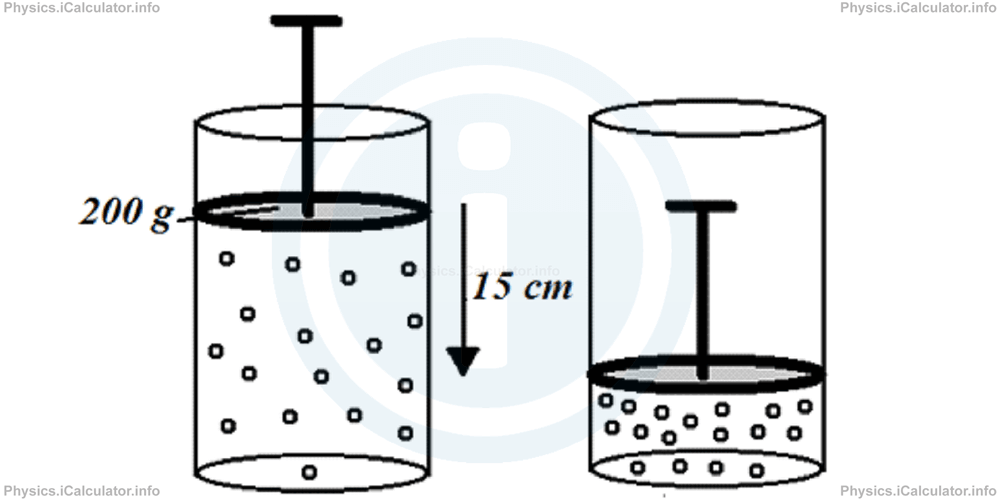
Solution 2
The work done by the external force to the system is
= m × g × ∆x
= 0.2 kg × 10 N/kg × 0.15 m
= 0.3 J
This means the work done by the system on the surroundings is - 0.3 J. Therefore, since no change in temperature does occur (the process is very fast), we obtain
= -(-0.3)J
= 0.3 J
This result means the internal energy of the gas increases by 0.3 J during this process. Thus, the final internal energy of the gas becomes
= 1.4 J + 0.3 J
= 1.7 J
II. Constant volume processes.
If the volume of a thermodynamic system remains constant for any reason, there is no work done on or by the system. This occurs when we fix the piston in an unmovable position. In such a case, all heat energy supplied to or removed by the system contributes in the increase (decrease) of its internal energy.
Mathematically, we can write:
Thus, if heat is absorbed by a system (that is, if Q is positive), its internal energy increases. Likewise, if heat is lost during the process (that is, if Q is negative), the internal energy of the system decreases.
Example 3
2500 J of heat energy is removed from a closed room. What was the initial internal energy of the room if the final internal energy at the end of process becomes 4600 J?
Solution 3
Since the room is closed, this is a thermodynamic process at constant volume. Hence, we can write:
= Ufinal - Uinitial
Since the heat is removed from the system, Q is negative (Q = -2500 J). Also, we have Ufinal = 4600 J. Thus, we obtain:
Uinitial = 4600 J + 2500 J
= 7100 J
III. Cyclical processes.
In some thermodynamic processes, the system parameters return to their original values after experiencing a number of changes in heat and work. We say the system is restored to its initial state. These are known as cyclical processes. During such processes, no change in the internal energy does occur (ΔU = 0).
Mathematically, we can write
Example 4
How many cm above the original position a 6 kg piston will raise when 12 J of heat energy is supplied to the gas inside the cylinder? Assume there is no change in the gas internal energy during the entire process. Take g = 10 N/kg.
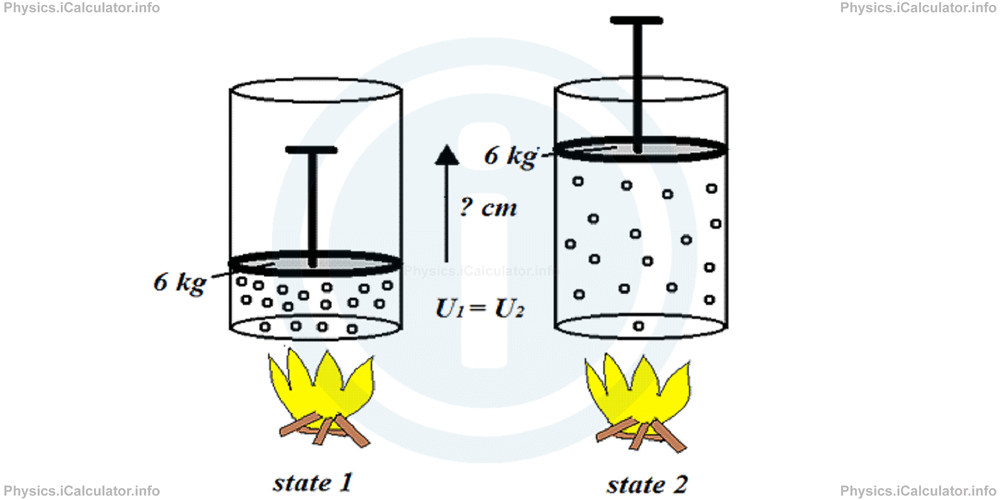
Solution 4
This is a cyclical process in which no change in the internal energy of the system does occur. Therefore, we can write
= F × ∆x
= m × g × ∆x
Thus, the piston will raise by
= 12 J/6 kg × 10 N/kg
= 12 J/60 N
= 0.2 m
= 20 cm
IV. Free expansion processes.
In such processes, there is no change in the internal energy of the system and also there is no heat supplied to or removed by the system. An example in this regard is a system composed by two glass containers connected through a narrow tube with a valve at middle as shown in the figure.
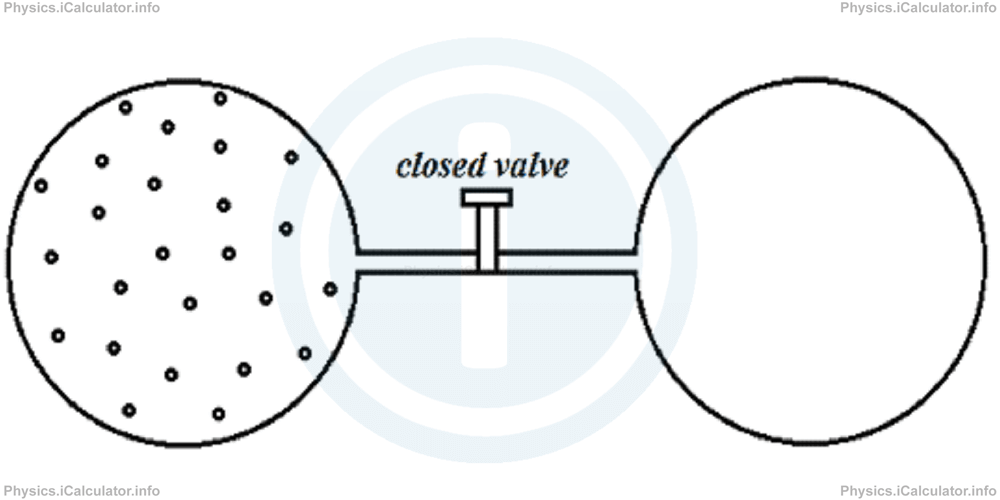
When we open the valve, the gas flows through the empty container. No work is done in this process, as there is no pressure to overcome in the empty container.
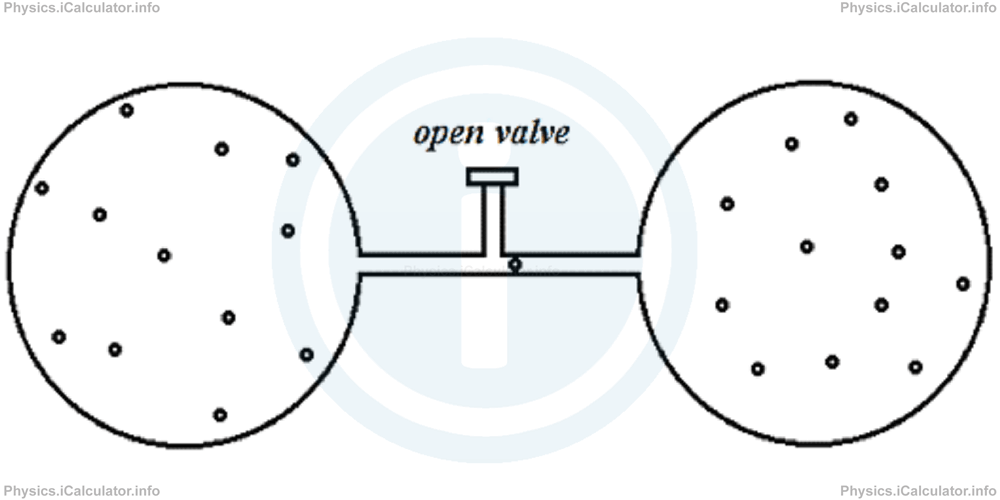
Therefore, we have Q = 0, ΔU = 0 and W = 0. The reason why this process is called "free expansion" is because the gas flow occurs naturally, without any intervention from outside.
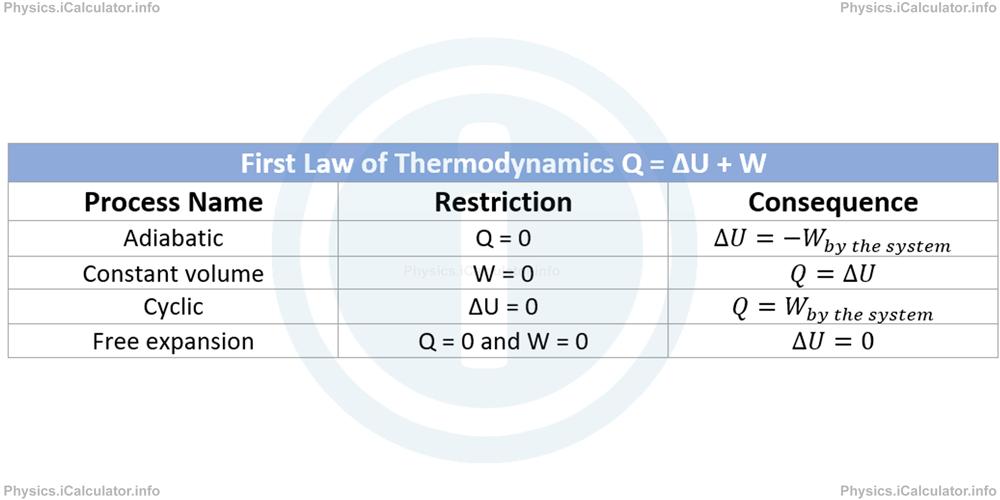
The following table includes all restrictions and consequences in the quantities involved in the four special thermodynamic processes discussed above.
You have reached the end of Physics lesson 13.5.6 Special Cases of the First Law of Thermodynamics. There are 6 lessons in this physics tutorial covering The First Law of Thermodynamics, you can access all the lessons from this tutorial below.
More The First Law of Thermodynamics Lessons and Learning Resources
Whats next?
Enjoy the "Special Cases of the First Law of Thermodynamics" physics lesson? People who liked the "The First Law of Thermodynamics lesson found the following resources useful:
- Special Feedback. Helps other - Leave a rating for this special (see below)
- Thermodynamics Physics tutorial: The First Law of Thermodynamics. Read the The First Law of Thermodynamics physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: The First Law of Thermodynamics. Print the notes so you can revise the key points covered in the physics tutorial for The First Law of Thermodynamics
- Thermodynamics Practice Questions: The First Law of Thermodynamics. Test and improve your knowledge of The First Law of Thermodynamics with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: The Kinetic Theory of Gases. Ideal Gases
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "The First Law of Thermodynamics" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator