Menu
Physics Lesson 13.5.1 - Useful Definitions in Thermodynamics
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Useful Definitions in Thermodynamics, this is the first lesson of our suite of physics lessons covering the topic of The First Law of Thermodynamics, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Useful Definitions in Thermodynamics
Before discussing the First Law of Thermodynamics, it is appropriate to define some quantities we will encounter during the explanation of this tutorial but also of the entire section.
a) System and Surroundings
In thermodynamics, a system is a definite group of objects or substances that we choose to study. We must specify well what objects and properties are part of a given system and what are not. Everything out of the system's boundary belongs to the surroundings of the system.
For example, a glass of water, a cylinder filled with gas or a human body - all of these can be considered as thermodynamic systems.
The choice of a system in thermodynamics is an arbitrary action. However, a clear definition of the system boundary is very important. For example, let's consider a gas trapped in a vertical cylinder which has a moveable piston on its upper part. The piston is initially fixed and then it is allowed to fall down freely. Eventually it stops falling due to the gradual increase in gas pressure trapped below it. We can use two different approaches to analyze this situation. Each approach gives different values for the quantities involved. For example:
I. We can choose to consider as a system only the gas in the cylinder as shown in the figure.
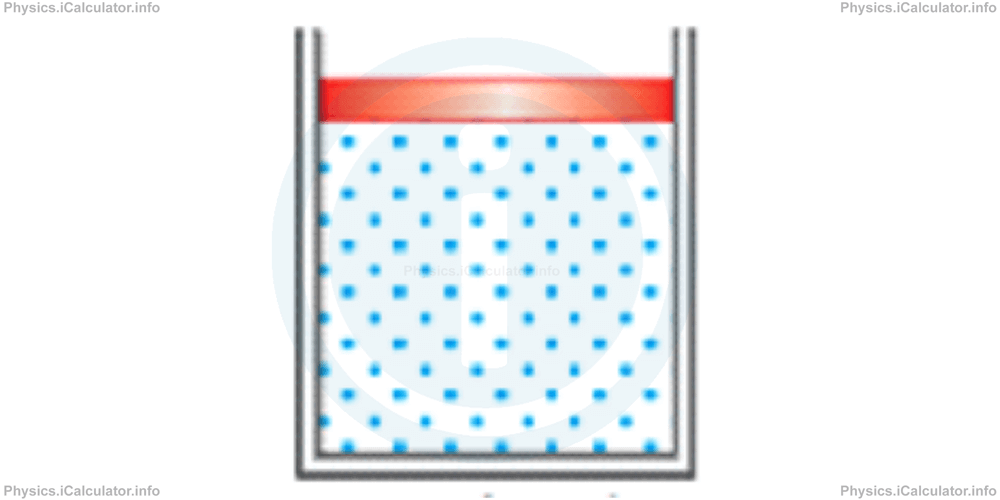
In this case, we say: "the energy of our system has increased", because the potential energy lost by the falling piston (which is not part of the chosen system) is transferred to the gas.
II. We can choose to consider the gas and the cylinder - piston mechanism as the system.
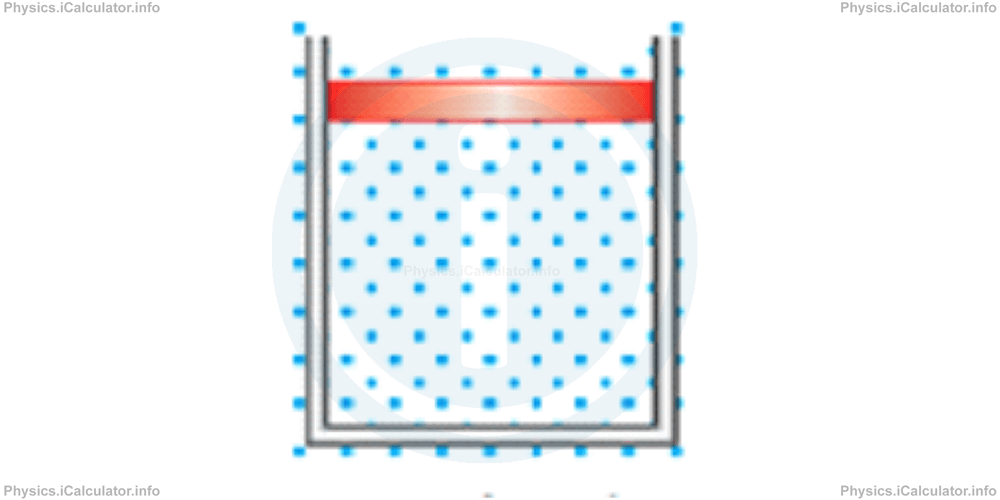
In this case, we say: "the energy of the system has not changed", because the energy lost by one part of the system (potential energy of the piston is decreased) is gained by another part of it (the internal energy of the gas is increased).
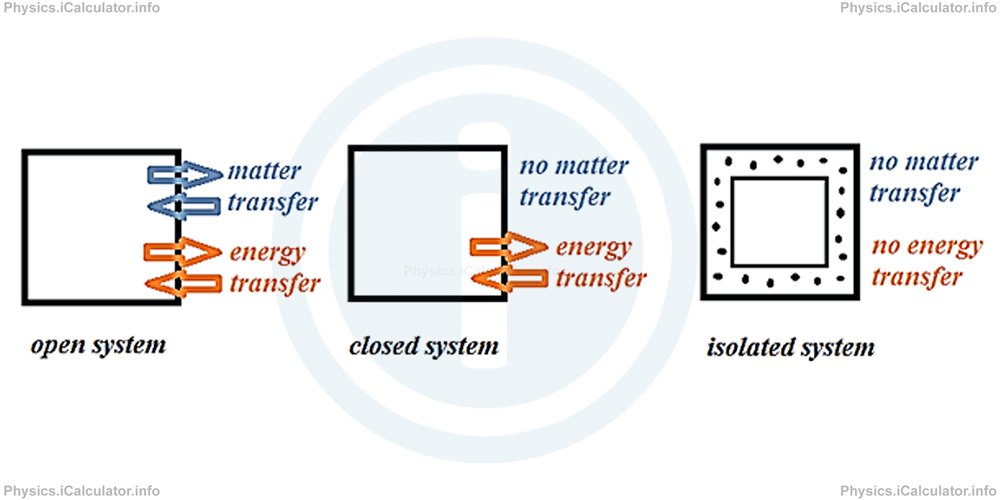
There are three kinds of thermodynamic systems:
- Closed systems, in which there is no matter transfer between the system and its surroundings. The example with the cylinder filled with gas shown earlier is an example of a closed system, as the gas cannot escape from the cylinder.
- Isolated systems, in which neither matter nor energy is transferred between the system and its surroundings. For example, a vacuum flask can be considered as an isolated system at a certain extent because no heat exchange takes place between the inner part of the vacuum flask and the surrounding air.
- Open systems, in which both matter and energy transfer can occur between the system and the surroundings. For example, an open bottle filled with hot water is considered an open system as water evaporates and gives off energy to the surroundings.
The diagram below shows schematically the three types of thermodynamic systems:
b) State of a System
Each system has its own set of properties, which describe the state of the given system. To describe a certain state of a system, we must choose the properties that describe in full the exact condition of it. For example, if we want to describe the state of a person, we must know a number of his properties such as his height, hair and eye colour, age, occupation, etc. Similarly, we must know a number of properties in a gas sample in order to describe its thermodynamic state. There are only three variables used to describe a thermodynamic system. They are pressure, volume and temperature. Specified values of P, V and T give the state of a gas.
State variables are those variables that always have the same value when the system is in a given state. Pressure, volume and temperature are state variables for a gas. Internal energy is also a state variable, as we will see later in this tutorial.
Equilibrium state for a gas implies that temperature (and therefore pressure and density) of the gas sample has the same value in all parts of its volume. When a gas sample is in equilibrium, its microscopic parameters such as the individual speeds of molecules will change over time. On the other hand, macroscopic parameters involving average effects of many molecules such as pressure, temperature and volume stay constant with time. Look at the figure.
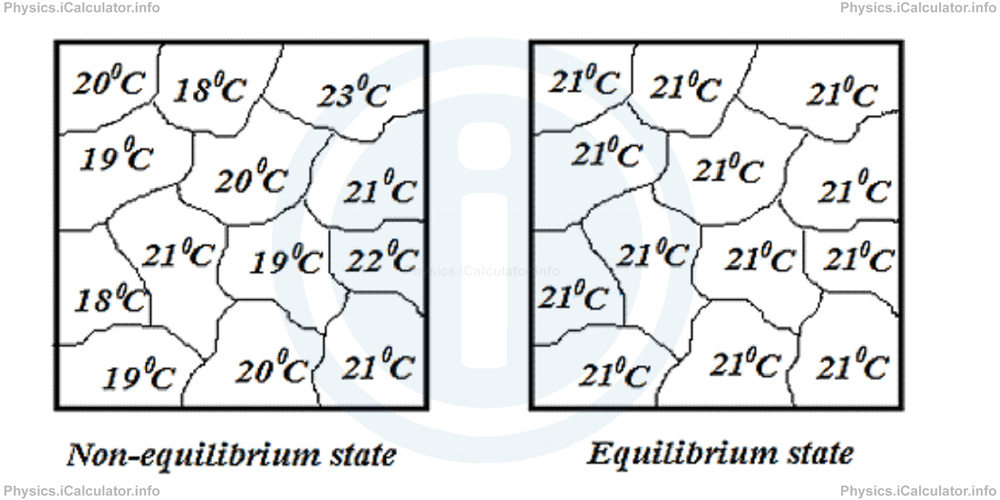
In the first figure, the average kinetic energy of different molecular groups have different values because temperatures are different. Therefore, the gas is turbulent and the temperature of the gas is not well defined. This means the substance is not in equilibrium state. On the other hand, the second figure shows a substance in equilibrium state as all molecular groups have the same temperature and therefore, their average kinetic energy is equal. This mean the gas is not turbulent.
When a gas sample is in equilibrium, it has definite values of pressure and temperature. In other words, pressure or temperature of a gas have well defined values only when the gas sample is in an equilibrium state.
Classical Thermodynamics deals only with systems in state of equilibrium. This means the Laws of Thermodynamics are valid only when applied to systems in thermal equilibrium. Non-equilibrium states are discussed only in more advanced sub-branches of thermodynamics.
c) Thermal processes
Any change from one thermodynamic state to another is called a thermal process. In this tutorial, we will discuss only slow processes because they are easy to analyze. The difference between slow and fast processes is shown in the example below.
Let's consider a cylinder filled with gas which is compressed to one-third of the original volume as shown in the figure.
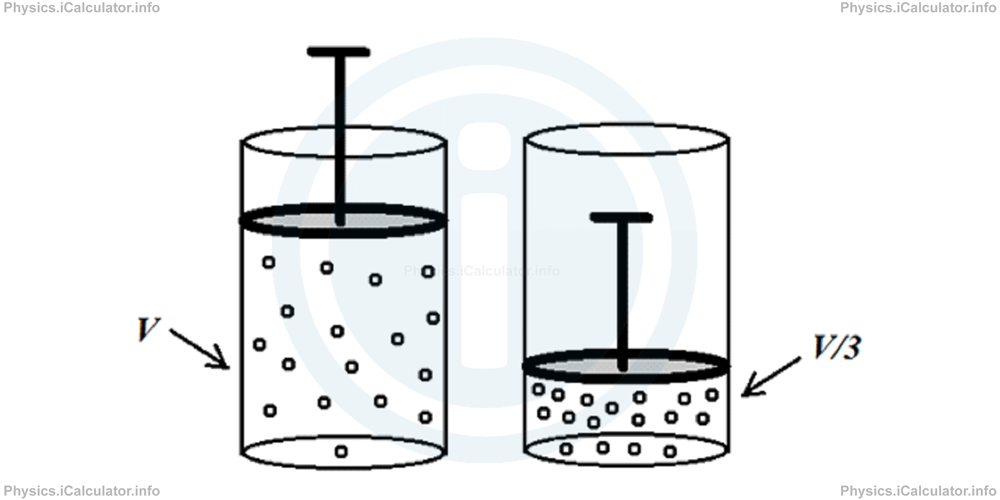
There are two possible paths to follow in performing this action.
1- By pushing down the piston very gently. In this case, the homogeneity of the gas is maintained during the entire process. As a result, we can plot a very accurate pressure vs volume graph, in which not only the initial and final values of pressure and volume, but also all their values in the intermediate stages are clearly defined.
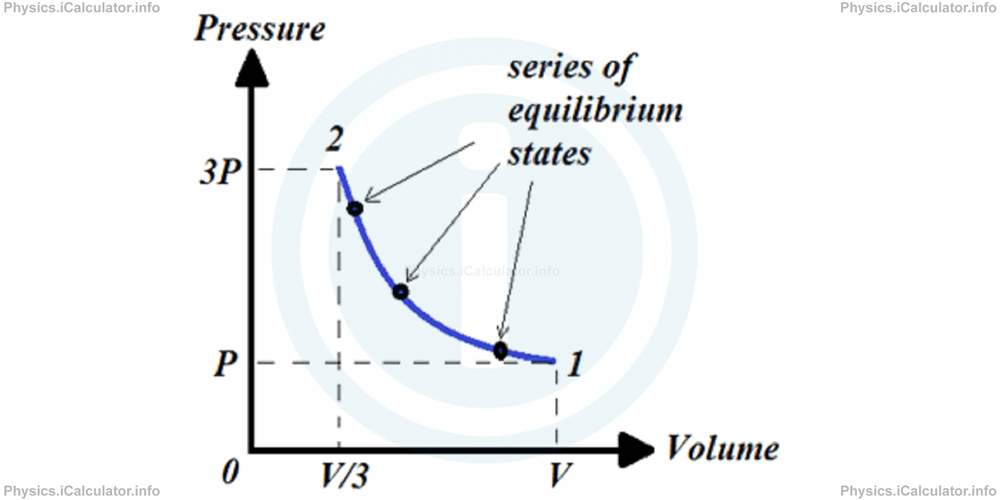
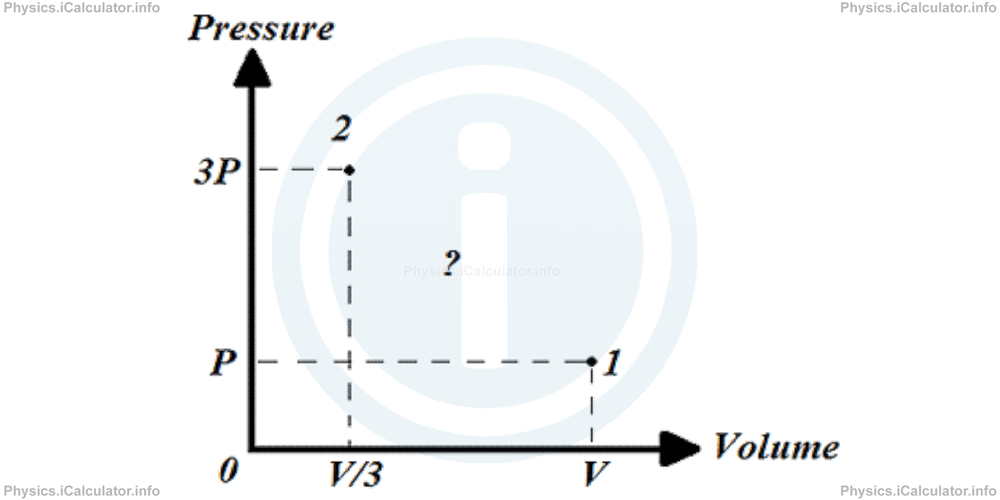
2- By pushing the piston very rapidly. In this case, the homogeneity of the gas is not maintained although the initial and the finale values of pressure and volume will still are well defined as in the first case. Now we cannot plot an accurate P vs V graph to show the intermediate values. At most, we can plot a graph like this:
You have reached the end of Physics lesson 13.5.1 Useful Definitions in Thermodynamics. There are 6 lessons in this physics tutorial covering The First Law of Thermodynamics, you can access all the lessons from this tutorial below.
More The First Law of Thermodynamics Lessons and Learning Resources
Whats next?
Enjoy the "Useful Definitions in Thermodynamics" physics lesson? People who liked the "The First Law of Thermodynamics lesson found the following resources useful:
- Definitions Feedback. Helps other - Leave a rating for this definitions (see below)
- Thermodynamics Physics tutorial: The First Law of Thermodynamics. Read the The First Law of Thermodynamics physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: The First Law of Thermodynamics. Print the notes so you can revise the key points covered in the physics tutorial for The First Law of Thermodynamics
- Thermodynamics Practice Questions: The First Law of Thermodynamics. Test and improve your knowledge of The First Law of Thermodynamics with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: The Kinetic Theory of Gases. Ideal Gases
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "The First Law of Thermodynamics" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator