Menu
Physics Lesson 13.10.5 - Heat Engines
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Heat Engines, this is the fifth lesson of our suite of physics lessons covering the topic of Entropy and the Second Law of Thermodynamics, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Heat Engines
Heat engines operate by converting heat into mechanical energy, i.e. they produce motion from heat energy. Car motors, diesel engines, steam turbines and steam power plants are all examples of heat engines.
A heat engine uses a gas at high pressure to push against a piston. For this, it needs a source of thermal energy to heat up the gas inside the cylinder. These thermal energy sources originally are in another form, most commonly as chemical energy of fuels. Therefore, the chain of energy conversions in heat engines is
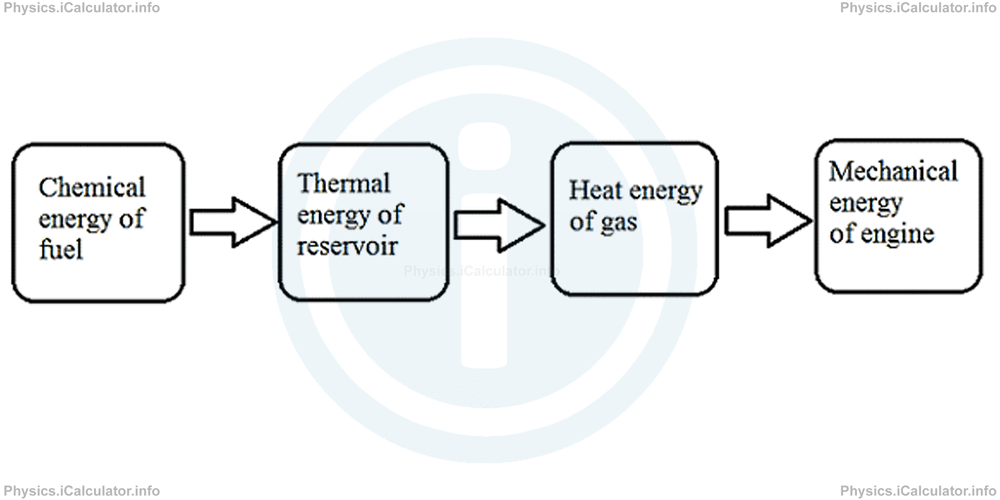
In simple terms, a heat engine absorbs heat energy from a source (called "hot reservoir") at high temperature, then it converts part of this energy into useful work and expels the rest outside the system (in the surroundings). Such a medium is at lower temperature than the system and is known as "cold reservoir" or "heat sink".
A simplified scheme of a heat engine operation is shown in the figure below.
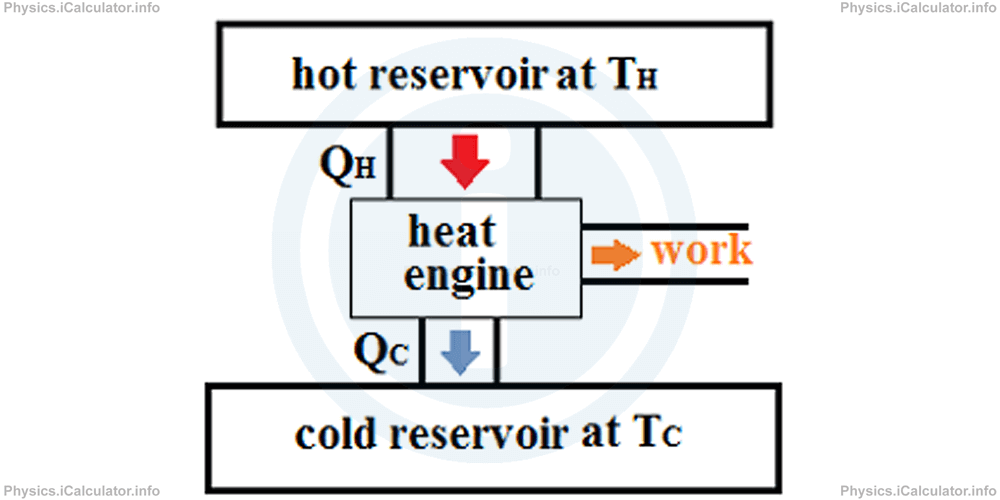
QH represents the amount of heat supplied to the heat engine from a source at high temperature,
QC represents the amount of heat energy given off by the heat engine to a low temperature reservoir, usually the atmosphere,
TH is the absolute temperature of the hot reservoir,
TC is the absolute temperature of the cold reservoir, and
W is the mechanical work done by the heat engine.
The cold reservoir is necessary as otherwise, the heat engine would heat up continuously and eventually, it would melt down. Also, both QH and QC are taken as positive.
For continuous operation, a heat engine must operate in cycles, i.e. it must cool down to the initial temperature before starting the new cycle.
Efficiency of Heat Engines
Efficiency of heat engines is conceptually similar to the efficiency of all mechanical devices, i.e. it represents the ratio of output and input energy of engine. Its formula is
where W is the work done by the engine, which represents the output (or useful) energy and Q is the input (or total) heat energy supplied by the source.
Example 2
400 g of fuel are consumed by a 65 kW heat engine to make a car travel for 4 minutes. The fuel has a calorific value of 45 megajoules/kg. What is the efficiency of the engine? The formula of heat energy released by a burning fuel is
where m is the mass of fuel and q is its calorific value.
Solution 2
Work (or useful energy) is obtained by multiplying power and time. Since 65 kW = 65 000 W and 4 minutes = 240 seconds, we have
= 65 000 W × 240 s
= 15 600 000 J
The heat energy released by the burning fuel (which represents the total or input energy of the source) is
= 0.4 kg × 45 000 000 J/kg
= 18 000 000 J
Therefore, the efficiency of this heat engine is
= 15 600 000 J/18 000 000 J × 100%
= 86.7 %
This means 86.7% of the original heat energy supplied by the source is used to do work while the rest 13.3% is wasted energy that goes into the environment through the cold reservoir during cyclic processes.
Now, we can provide a more formal definition for entropy, which is based on the quantities involved in application of the Second Law of Thermodynamics in thermal engines:
Entropy is a thermodynamic quantity representing the unavailability of a system's thermal energy for conversion into mechanical work, often interpreted as the degree of disorder or randomness in the system.
You have reached the end of Physics lesson 13.10.5 Heat Engines. There are 6 lessons in this physics tutorial covering Entropy and the Second Law of Thermodynamics, you can access all the lessons from this tutorial below.
More Entropy and the Second Law of Thermodynamics Lessons and Learning Resources
Whats next?
Enjoy the "Heat Engines" physics lesson? People who liked the "Entropy and the Second Law of Thermodynamics lesson found the following resources useful:
- Heat Engine Feedback. Helps other - Leave a rating for this heat engine (see below)
- Thermodynamics Physics tutorial: Entropy and the Second Law of Thermodynamics. Read the Entropy and the Second Law of Thermodynamics physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Entropy and the Second Law of Thermodynamics. Print the notes so you can revise the key points covered in the physics tutorial for Entropy and the Second Law of Thermodynamics
- Thermodynamics Practice Questions: Entropy and the Second Law of Thermodynamics. Test and improve your knowledge of Entropy and the Second Law of Thermodynamics with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Temperature. The Zeroth Law of Thermodynamics
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Entropy and the Second Law of Thermodynamics" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator