Menu
Physics Lesson 13.10.6 - Carnot Engine
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Carnot Engine, this is the sixth lesson of our suite of physics lessons covering the topic of Entropy and the Second Law of Thermodynamics, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Carnot Engine
The Second Law of Thermodynamics implies that no perfect machine can exist i.e. the efficiency of heat engines cannot be 1. This means there is some heat lost during the process.
The question arisen here is: How to construct a machine with the maximum efficiency possible and what is the value of this maximum efficiency?
From experiments, it is proven that the type of engine that produces the maximum efficiency is the "Carnot Engine", named after the famous French scientist Sadi Carnot. A Carnot engine produces a definite reversible cycle which has a maximum possible efficiency between two given heat reservoirs with different temperatures. In other words, a Carnot cycle is the most efficient cycle that can theoretically exist.
A schematic-graphical representation of a Carnot cycle is shown in the figure below.
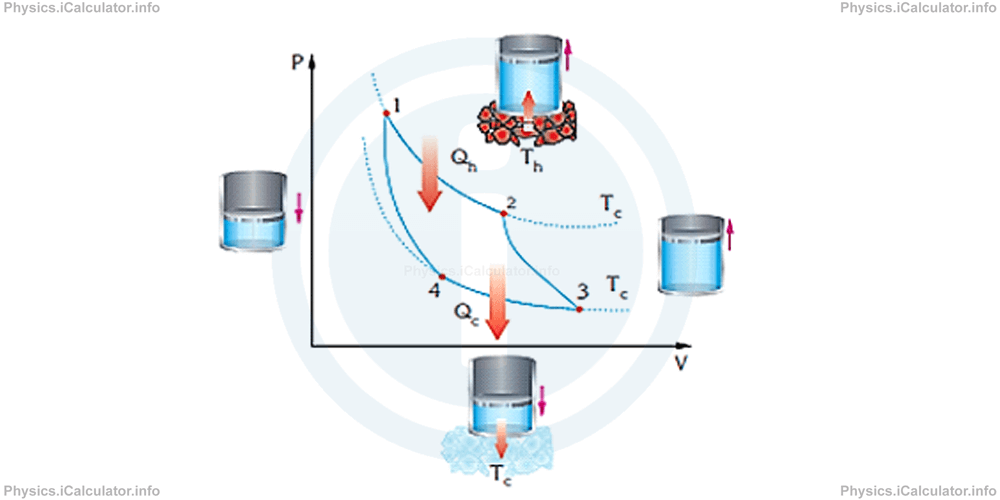
From the graph above, you can see that a Carnot cycle consists in two adiabatic and two isothermal processes. All these processes are reversible.
Based on the figure above, we can say that:
- Processes 2-3 and 4-1 are adiabatic. This means no heat is transferred between various parts of the engine.
- Process 1-2 represents an isothermal expansion. During this process, the gas absorbs some heat (QH) from a hot source with temperature TH.
- During the process 3-4, gas is compressed isothermally, giving of some heat (QC) to a cold reservoir with temperature TC.
The figure below shows what happens to the gas produced by the heat source during a Carnot cycle.
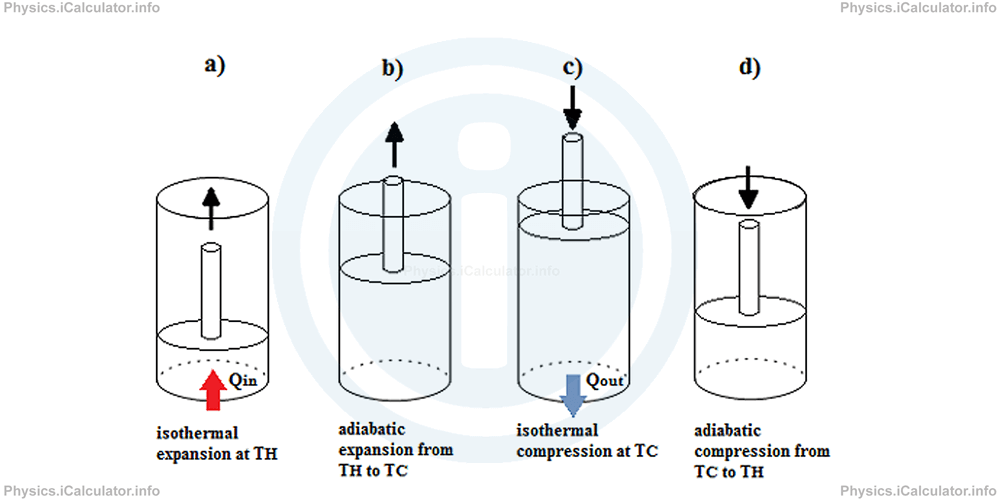
For the same difference in temperatures, the Carnot engine is the most efficient among all the other types of engines. However, a Carnot engine is more a theoretical model than a reality. As we have stated earlier, it is quite impossible to obtain reversible cycles during a thermal process. Carnot engine however, is very useful in determining the maximum efficiency a heat engine can have. Hence, we can say the efficiency of a real heat engine operating between the temperatures TH and TC is lower than that of a Carnot engine with the same range of temperatures. Thus, we can write
Two factors decrease the efficiency of a real engine. They are:
- A real machine does not operate according a Carnot cycle
- Friction between various parts of the engine decreases further the efficiency
The equation used to find the efficiency in a Carnot engine is
= TH/TH - TC/TH × 100%
= 1 - TC/TH × 100%
Example 3
A Carnot engine operates between the minimum and maximum temperature of water. What is its efficiency of this engine?
Solution 3
Water exists in liquid state between 0°C and 100°C. The first represents the temperature of cold reservoir (TC) and the other, that of the hot reservoir (TH). When converted into Kelvin scale, we obtain TC = 0 + 273 = 273 K and TH = 100 + 273 = 373 K.
Therefore, we obtain for the efficiency of this Carnot engine:
= 373 K - 273 K/373 K × 100%
= 100 K/373 K × 100%
= 26.8%
This is a small value, which in reality decreases further when we consider the above-mentioned factors. Therefore, most engines operate between higher ranges of temperatures, in order to increase the numerator of fraction, which brings in an increase in the engine's efficiency.
You have reached the end of Physics lesson 13.10.6 Carnot Engine. There are 6 lessons in this physics tutorial covering Entropy and the Second Law of Thermodynamics, you can access all the lessons from this tutorial below.
More Entropy and the Second Law of Thermodynamics Lessons and Learning Resources
Whats next?
Enjoy the "Carnot Engine" physics lesson? People who liked the "Entropy and the Second Law of Thermodynamics lesson found the following resources useful:
- Carnot Engine Feedback. Helps other - Leave a rating for this carnot engine (see below)
- Thermodynamics Physics tutorial: Entropy and the Second Law of Thermodynamics. Read the Entropy and the Second Law of Thermodynamics physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Entropy and the Second Law of Thermodynamics. Print the notes so you can revise the key points covered in the physics tutorial for Entropy and the Second Law of Thermodynamics
- Thermodynamics Practice Questions: Entropy and the Second Law of Thermodynamics. Test and improve your knowledge of Entropy and the Second Law of Thermodynamics with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Temperature. The Zeroth Law of Thermodynamics
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Entropy and the Second Law of Thermodynamics" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator