Menu
Physics Lesson 13.4.3 - Methods of Heat Transfer
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Methods of Heat Transfer, this is the third lesson of our suite of physics lessons covering the topic of Calorimetry (Heat Transfer), you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Methods of Heat Transfer
Heat is transferred from one place to another through four basic methods. They are:
a) Conduction
As stated earlier, particles in hot objects vibrate faster than in cold objects. When we place in contact a hot and a cold object, heat flows through the collision of particles from the hot to the cold object. This method of heat transfer is known as conduction.
By definition, conduction is the method of heat transfer through the collision of particles.
In simpler words, conduction is the heat flow between objects through direct contact.
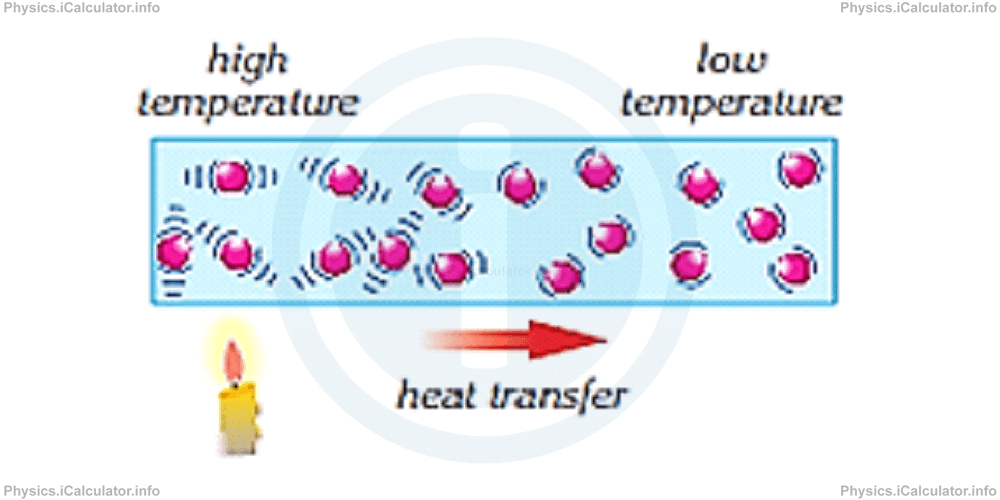
Not all object conduct the heat equally. Some materials conduct heat better than the others. As explained earlier, such materials as known as good conductors of heat. For example, most metals are good conductors of heat.
On the other hand, some materials conduct very poorly the heat. They are known as bad (poor) conductors of heat. Wood, glass, cork, plastics, air, vacuum etc., are all bad conductors of heat.
If you touch a wooden and a metal object inside a room in a cold winter day, the metal feels colder. This is not because metal has a lower temperature than wood. These two materials have the same temperature, as both they are within the same room. The reason why metal feels colder is because it is a better heat conductor than wood. Thus, when we touch the materials, heat flows at a higher rate from our body to metal than from our body to the wooden object. This process of heat flow takes place only in one direction - from our body to the material and not vice-versa because our body has a normal temperature of 37°C, which is much higher than that of a room temperature in winter.
Conduction is a method of heat transfer that does not involve any matter transfer between objects. However, matter must be present during this process, as it is needed to transmit the heat through collision between particles.
There is a mathematical equation, which allows us to calculate the rate of heat flow through a solid. Thus, the heat rate (heat per unit time) flowing through a solid object of surface A and thickness d during which the temperature changes from T1 to T2 is
where K is a quantity known as the coefficient of thermal conduction, measured in [W/m × K]. It depends on the characteristics of material.
Example 6
What is the amount of heat absorbed by a 40 cm2 copper plate of 4 mm thickness if during 2 minutes the temperature of copper increases from 20°C to 30°C? Take the coefficient of thermal conduction for copper equal to 385 W/m × K.
Solution 6
In this problem, there are the following clues:
A = 40 cm2 = 0.004 m2
d = 4 mm = 0.004 m
t = 2 min = 120 s
T1 = 20°C
T2 = 30°C
K = 385 W/m × K
Q = ?
We have:
Q = K × A × (T2 - T1 )/d × t
= 385 × 0.004 × (30 - 20) × 120/0.004
= 462 000 J
b) Convection
When we mix two liquids or gases, the heat is distributed equally throughout the entire volume of mixture. This method of heat transfer is known as convection.
By definition, convection is the method of heat transfer through the circulation of fluid itself.
This process involves both heat and matter transfer. Obviously, matter must be present during convection.
There are two types of convection: natural and forced convection. Wind is an example of natural convection as it occurs without the intervention of humans. On the other hand, air flowing when we turn on an electric fan is an example of forced convection as this process involves human made tools to produce air circulation.
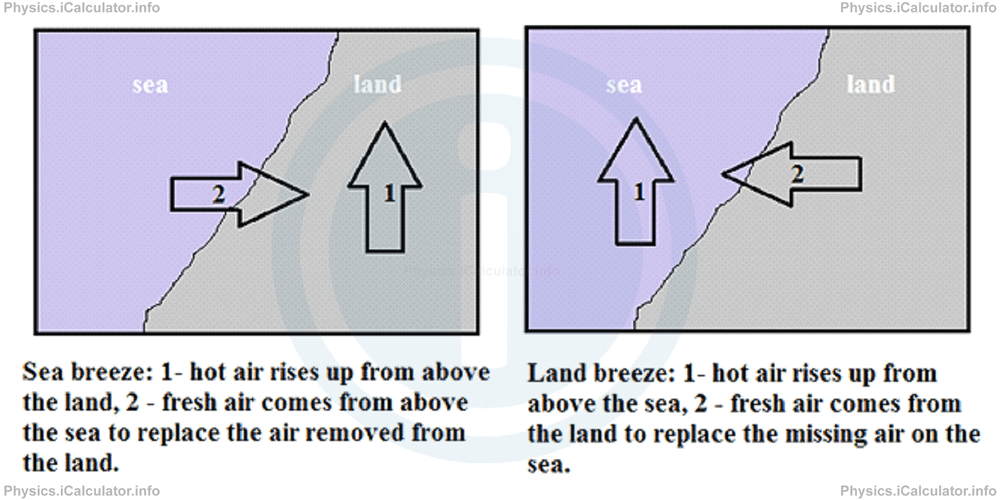
Coastal breeze is another example of natural convection. During the day, the sand heats up faster than water (water is one of substances with the highest specific heat capacity; this means water heats up and cools down much slower than other substances). Therefore, the air above the sand expands more than that above water. Such expansion causes a decrease in air density above the sand (air becomes rarer than before). The fresh air coming from regions above the sea replaces the missing air on the land regions. In this way, a slight wind called sea breeze is produced.
During the night, the reverse process does occur. Water is warmer than sand because it cools down slower. As a result, the air above water surface expands and it is replaced by fresh air coming from above the sandy regions. Therefore, a slight wind called land breeze is produced.
We can find the rate of heat flow through convection using the equation
where k is the coefficient of thermal convection measured in [W/m2K] and A is the area involved.
Example 7
A fluid flows over a plane surface 2 m by 2 m. The surface temperature is 40°C, the fluid temperature is 30°C and the convective heat transfer coefficient is 1000 W/m2K. What is convective heat transfer between the surface and the fluid in 10 seconds?
Solution 7
Clues:
A = 2 m × 2 m = 4 m2
T1 = 30°C
T2 = 40°C
k = 1000 W/m2
t = 10 s
Q = ?
Applying the equation of convective heat transfer
we obtain after rearranging and substituting the known values:
= 1000 × 4 × 10 × (40 - 30)
= 400 000 J
c) Radiation
Radiation is the third method of heat transfer. By definition, radiation is the method of heat transfer by means of electromagnetic waves.
Most radiation comes to Earth from the Sun. Radiation does not involve any matter transfer; it only transfers energy. Also, matter presence is not necessary in this process, as most radiation travels through vacuum.
When radiation falls on an object, it is partly reflected, partly transmitted and partly absorbed. The absorbed heat causes the molecules of the object to vibrate more than before, so it becomes hot.
Some objects emit radiation more than the others; they are known as black bodies. This is because black bodies absorb radiation more than other bodies. As a result, they accumulate a lot of energy. As a result, when black bodies become hot, they emit large amounts of this radiation to the surroundings.The radiation energy per unit time from a black body is proportional to the fourth power of the absolute temperature and can be expressed with Stefan-Boltzmann Law as
where σ is a quantity known as Stefan-Boltzmann constant. It has a value of 5.6703 × 10-8 W/m2K4. T is the absolute temperature of the black body in Kelvin degree and A is the area of the emitting body.
Here, a black body is more an idealization than reality; it is quite impossible to find a perfect black body that is able to absorb all radiation falling on it and then, emitting all this amount of heat to the surroundings. For objects other than ideal black bodies ("gray bodies") the Stefan-Boltzmann Law can be expressed as
where ε is a dimensionless quantity between 0 and 1 (one for black bodies) called emissivity coefficient of the object.
Example 8
A grey body of emissivity equal to 0.4 has an emitting surface of 3 m2. How many joules of heat energy per second does this body emit when it is at 27°C?
Solution 8
We have:
ε = 0.4
A = 3 m2
T = 27°C = 300 K = 3 × 102 K
σ = 5.6703 × 10-8 W/m2K4
Q/t = ?
Applying the Stefan-Boltzmann Law for grey bodies
we obtain after substitutions
= 551 J/s
= 551 W
When a hot object of temperature TH radiates heat energy to its cool surroundings of temperature TC, the rate of net radiation heat loss can be expressed as
where AH is the area of the hot emitting body.
Example 9
A hot object at 127°C radiates heat energy at 2000 J/s to the surroundings, whose temperature is 27°C. The object has a coefficient of emissivity equal to 0.8. What is the area of the hot object in 2>? Take σ = 5.6703 × 10-8 W/m2K4.
Solution 9
Clues:
TH = 127°C = 400 K = 4 × 102 K
TC = 27°C = 300 K = 3 × 102 K
Q/t = 2000 J/s = 2 × 103 J/s = 2 × 103 W
ε = 0.8
σ = 5.6703 × 10-8 W/m2K4
A = ?
Applying the equation
we obtain after rearranging and substitutions:
= 2 × 103/0.8 × 5.6703 × 10 - 8 × [(4 × 102) - (3 × 102)]4
= 2 × 103/4.53624 × 10-8 × (256 - 81) × 108
= 2000/794
= 2.52 m2
d) Evaporation
Evaporation is sometimes regarded as the fourth method of heat transfer. By definition, evaporation is a method of heat transfer through heat removal from a hot body by means of change in state of a covering liquid.
When a liquid evaporates from a hot surface, it takes off heat energy from it. As a result, the surface gets colder. For example, when we sweat in summer it is because our body wants to get rid of the excessive heat it has accumulated due to the thermal processes occurred when chemical reactions take place in food we consume. Many chemical reactions are associated with heat release. Our body feels comfortable with a difference in temperature of 10-15°C with the surroundings. In this case, heat energy flows naturally from our body to the surrounding air. However, in hot days, this difference in temperature (otherwise known as temperature gradient) is much smaller, in some cases it even becomes negative (the surrounding air is hotter than the human body). As a result, the body does not cool down properly. At this point, the evaporation process comes to help, as the body produces sweat and therefore, the excessive heat is removed from our body through evaporation of sweat. This is because the most energetic particles of liquid (sweat) get some heat energy from our body to make them pass in gaseous state and therefore, they leave the body surface.
Refrigerators and air conditioners use liquids with low boiling points to produce cooling effect through evaporation.
The rate of heat flow through evaporation by a human body is calculated through the equation
where h is the coefficient of heat transfer through evaporation, A is the area of the human body, ps is the pressure of water vapour near the skin and p0 is the pressure of water vapour in the environment air. The pressure of the water vapour near the surface of the skin depends on the humidity of the environment and the degree of sweating.
Example 10
The vapour pressure near the skin on a certain hot sunny day is 85 kPa while the air pressure is 100 kPa. Given that the body area of an adult is about 1.8 m2, calculate the heat lost due to evaporation in 2 minutes caused by the sweating process in the given conditions, if the coefficient of heat transfer through evaporation when the body is fully covered by sweat is 0.099 W/Pa × m2.
Solution 10
Clues:
ps = 25 kPa = 85 000 Pa
p0 = 100 kPa = 100 000 Pa
A = 1.8 m2
h = 0.099 W/Pa × m2
t = 2 min = 120 s
Q = ?
From the equation
we obtain after rearranging and substitutions:
= 0.099 × 1.8 × 120 × (85 000 - 100 000)
= -320 760 J
This is a big value, so the body needs immediately water and energy replacement through drinking and food.
The sign minus means the given amount of heat energy is leaving the body.
You have reached the end of Physics lesson 13.4.3 Methods of Heat Transfer. There are 3 lessons in this physics tutorial covering Calorimetry (Heat Transfer), you can access all the lessons from this tutorial below.
More Calorimetry (Heat Transfer) Lessons and Learning Resources
Whats next?
Enjoy the "Methods of Heat Transfer" physics lesson? People who liked the "Calorimetry (Heat Transfer) lesson found the following resources useful:
- Methods Feedback. Helps other - Leave a rating for this methods (see below)
- Thermodynamics Physics tutorial: Calorimetry (Heat Transfer). Read the Calorimetry (Heat Transfer) physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Calorimetry (Heat Transfer). Print the notes so you can revise the key points covered in the physics tutorial for Calorimetry (Heat Transfer)
- Thermodynamics Practice Questions: Calorimetry (Heat Transfer). Test and improve your knowledge of Calorimetry (Heat Transfer) with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: The First Law of Thermodynamics
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Calorimetry (Heat Transfer)" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator