Menu
Physics Lesson 13.4.1 - Heat Transfer
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Heat Transfer, this is the first lesson of our suite of physics lessons covering the topic of Calorimetry (Heat Transfer), you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Heat Transfer
As explained in the previous tutorial "Absorption of Heat. States of Matter. Change of State.", when two substances are placed in contact, there is a heat flow between them. The direction of heat flow is pre-determined; heat always flows from the hottest to the coldest object.
This "heat flow" does not necessarily imply any matter transfer; rather, it often takes place when the boundary atoms of the two objects collide with each other at different speeds. Such a collision only makes the slow vibrating molecules of the cold object vibrate faster due to their collision with the fast vibrating molecules of the hot object, as discussed in the previous tutorials.
However, in many cases there is also some matter transfer during the process of heat exchange between systems. This occurs for example when we mix some hot and cold water. The result will be an amount of warm water, whose mass is the sum of the individual masses. Obviously, it is expected that the warm water have an in-between temperature.
The general law of calorimetry states that:
During a heat exchange process between two objects of a thermodynamic system, the heat released by the hottest object is entirely gained by the coldest object if the system is isolated from the surroundings.
Mathematically, we can write
We can write for the heat released by the hottest object
= m1 × c1 × (t1-tf )
where m1 is the mass of the hottest object, c1 is its specific heat capacity, t1 is the initial temperature of the hottest object and tf is the final (common) temperature after the heat exchange process is completed.
On the other hand, we can write for heat gained by the coldest object
= m2 × c2 × (tf - t2 )
where m2 is the mass of the coldest object, c2 is its specific heat capacity, t2 is the initial temperature of the coldest object and tf is the final (common) temperature after the heat exchange process is done.
Pay attention to the expressions within the parenthesis. In the expression for the hottest object, we subtract the final temperature after heat exchange from the initial temperature as it gives a positive value, while in the expression for the coldest object we subtract the initial temperature before the contact from the final temperature in order to obtain a positive value. Therefore, combining the two above equations, we obtain the four equivalent equations (which basically represent the same thing):
or
or
or
Example 1
20 g water at 80°C is mixed with 30 alcohol at 10°C. Find the final temperature if no heat exchange with the surroundings does occur. Take the specific heat capacity of water as 1 cal/g°C and that of alcohol as 0.58 cal/g°C.
Solution 1
Using the notations introduced earlier in the theory, we write the following clues:
m1 = 20 g
m2 = 30 g
t1 = 80°C
t2 = 10°C
c1 = 1 cal/g°C
c2 = 0.58 cal/g°C
tf = ?
Applying the equation of heat exchange
we obtain after substituting the known values:
20 × (80 - tf ) = 17.4 × (tf - 10)
1600 - 20 × tf = 17.4 × tf - 174
37.4 × tf = 1774
tf = 1774/37.4
=47.4°C
We can plot a Temperature vs Heat graph like the one shown in the figure below to illustrate the phenomenon of heat exchange between two objects: one hot and one cold.
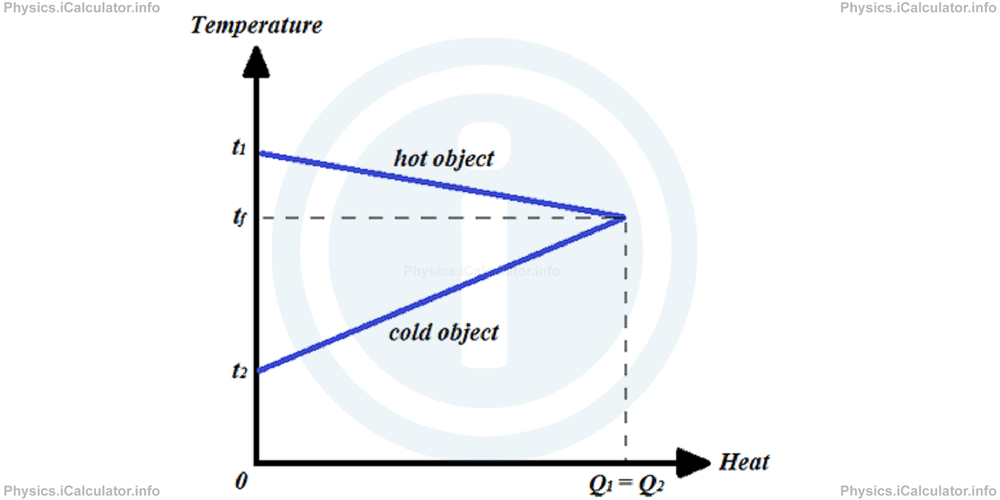
Example 3
Calculate the specific heat capacities of the two substances that exchange heat according the data provided in the graph below. Take the mass of the first substance as 2 kg and that of the second substance as 1 kg.
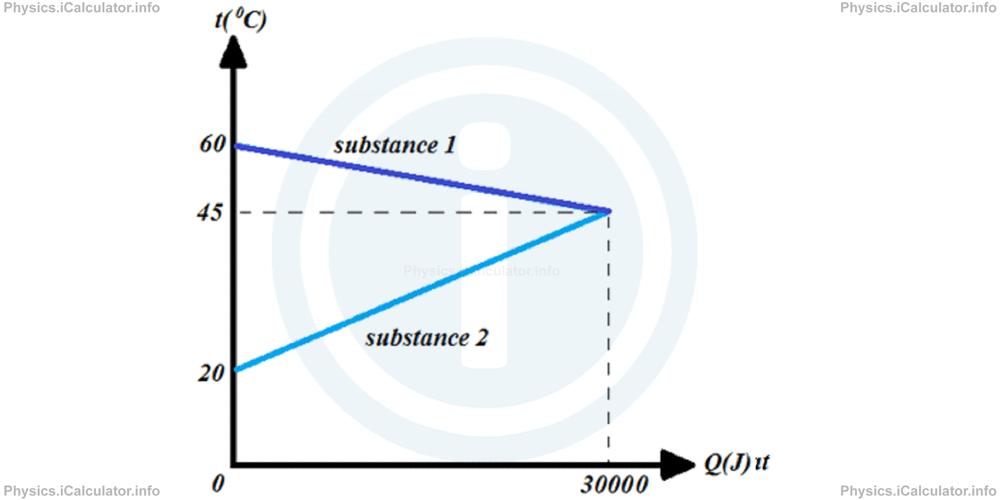
Solution 3
From the graph, we can extract the following clues:
t1 = 60°C
t2 = 20°C
tf = 45°C
Q1 = Q2 = 30 000 J
Also we have from the problem's words:
m1 = 2 kg
m2 = 1 kg
c1 = ?
c2 = ?
The heat released by the hot object is
Thus, the specific heat capacity of the hot object is
= 30 000/2 × (60 - 45)
= 1000 J/kg°C
The heat absorbed by the cold object is numerically the same as the heat released by the hot object. Thus, we can write:
Therefore, the specific heat capacity of the cold object is
= 30 000/1 × (45-20)
=1200 J/kg°C
You have reached the end of Physics lesson 13.4.1 Heat Transfer. There are 3 lessons in this physics tutorial covering Calorimetry (Heat Transfer), you can access all the lessons from this tutorial below.
More Calorimetry (Heat Transfer) Lessons and Learning Resources
Whats next?
Enjoy the "Heat Transfer" physics lesson? People who liked the "Calorimetry (Heat Transfer) lesson found the following resources useful:
- Heat Feedback. Helps other - Leave a rating for this heat (see below)
- Thermodynamics Physics tutorial: Calorimetry (Heat Transfer). Read the Calorimetry (Heat Transfer) physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Calorimetry (Heat Transfer). Print the notes so you can revise the key points covered in the physics tutorial for Calorimetry (Heat Transfer)
- Thermodynamics Practice Questions: Calorimetry (Heat Transfer). Test and improve your knowledge of Calorimetry (Heat Transfer) with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: The First Law of Thermodynamics
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Calorimetry (Heat Transfer)" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator