Menu
Physics Lesson 13.3.5 - Latent Heat. Specific Latent Heat of Fusion and Vaporization
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Latent Heat. Specific Latent Heat of Fusion and Vaporization, this is the fifth lesson of our suite of physics lessons covering the topic of Absorption of Heat, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Latent Heat. Specific Latent Heat of Fusion and Vaporization
Since there is no change in temperature during the phase change, we cannot use anymore the formula Q = m × c × ΔT to calculate the heat supplied to or removed from a substance during such a process, as ΔT = 0 and therefore, the value of Q would result zero as well. It means we must find other ways to calculate the heat during this process.
It is clear that the substance gains or loses heat during a phase change regardless its temperature remains constant. For this reason, this kind of heat - often forgotten to be considered during calculations - is known as latent (hidden) heat.
By definition, latent heat of fusion, Qf, is the amount of heat absorbed by a substance at the melting temperature in order to melt it completely.
Similarly, latent heat of vaporization, Qv, is the amount of heat absorbed by a substance at the boiling temperature in order to evaporate it completely.
Not all substances require the same amount of heat energy to change their state. Therefore, similarly as in the case of specific heat capacity, we introduce a new quantity known as the specific latent heat, L, which represents the amount of heat per kilogram mass needed to change the phase of a material. By definition,
Specific latent heat of fusion Lf is the amount of heat supplied to 1 kg of a substance in its melting temperature in order to make it melt completely.
Similarly, specific latent heat of vaporization Lv is the amount of heat supplied to 1 kg of a substance in its boiling temperature in order to make it evaporate completely.
Both specific latent heats are measured in Joules per kilogram, [J/kg]. The equation of latent heat for both cases therefore is
and
Example 3
What is the amount of heat absorbed by 200 g of solid lead at 600 K? Melting temperature of lead is 327°C and its specific latent heat of fusion is 22 900 J/kg.
Solution 3
600 K correspond to 327°C because T(K) = t°C + 273°. This is equal to the melting temperature of lead. Therefore, we have to use only the equation
to calculate the heat absorbed by lead during this stage. Also, we have to write the mass of lead in kilograms, i.e. m = 0.3 kg. Thus, we obtain after substituting the known values
=6370 J
for the heat absorbed by lead during the melting stage.
We can combine the formulae Q = m × c × Δ t and Q = m × L to calculate the amount of heat absorbed when the material is not at the melting or boiling temperature. Let's consider an example to clarify this point.
Example 4
What is the amount of heat absorbed by 50 g ice at -20°C in order to melt it completely? Take the melting (freezing) temperature of ice (water) equal to 0°C. Specific heat capacity of ice is 2100 J/kgK and the latent heat of fusion for ice is 334 000 J/kg.
Solution 4
The material passes into two stages during this event:
- The ice is "heated" from -20°C to 0°C. During this process, we must use the formula Q = m × c × Δ T.
- The ice is melted when it reaches the temperature 0°C. During this process, we must use the formula Q = m × Lf.
Given that m = 50 g = 0.05 kg, c = 4200 J/kgK, t1 = -20°C, t2 = 0°C and Lf = 334 000 J/kg, we write:
= m × c × (t2 - t1 ) + m × Lf
= 0.05 × 2100 × [0 - (-20)] + 0.05 × 334000
= 2100 J + 16700 J
= 18800 J
The graph below represents the relationship between heat and temperature in all possible stages.
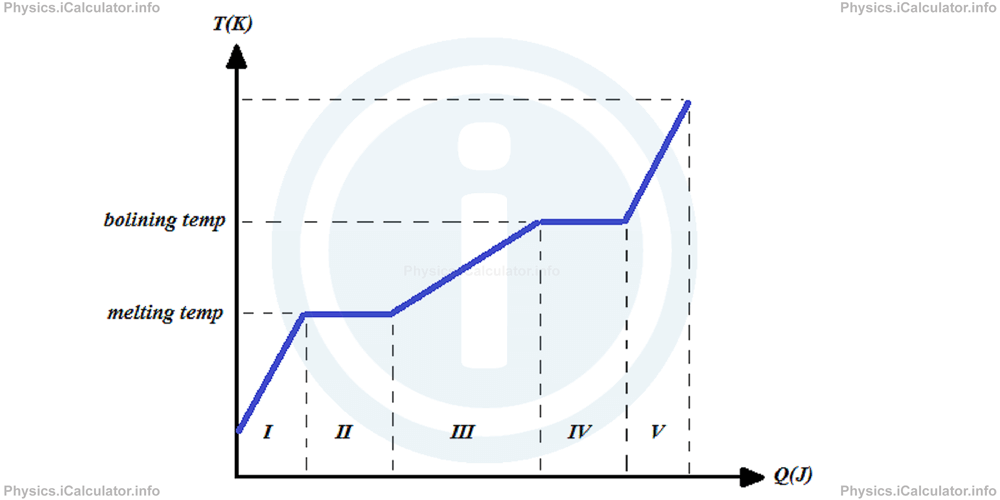
In part I of the graph, the substance is in solid state. We must use the formula Q = m × cs × ΔTs (s stands for solid) to calculate the heat absorbed by it during this stage.
In part II of the graph, the substance is melting. We must use the formula Q = m × Lf to calculate the heat absorbed by it during this stage.
In part III of the graph, the substance is in liquid state. We must use the formula Q = m × cl × ΔTl (l stands for liquid) to calculate the heat absorbed by it during this stage.
In part IV of the graph, the substance is boiling (evaporating at the highest rate). We must use the formula Q = m × Lv to calculate the heat absorbed by it during this stage.
In part V of the graph, the substance is in gaseous state. We must use the formula Q = m × cg × ΔTg (g stands for gas) to calculate the heat absorbed by it during this stage.
Remark!
- The specific heat capacities are not equal for the same material. For example, for water the value of specific heat capacity is 4186 J/kgK while for ice or water vapour, it is 2100 J/kgK. This is true for latent heats as well. For example, latent heat of fusion for ice is 334 000 J/kg and the latent heat of vaporization for water is 2 224 000 J/kg.
- If mass is given in grams and heat in calories, we can use the conversion factor 1 cal/g × °C = 4180 J/(kg × °C) in order to deal with smaller numbers.
- We can find the heat released by a hot object when it cools down by using the same procedure as when it is heated up. The only difference is that the heat value will result a negative number because t2 < t1 and therefore, Δt = t2 < t1 < 0.
Example 5
A 200 ml glass full of water initially at the environment temperature (20°C), is placed inside a refrigerator whose temperature is -10°C. Given that water has a density of 1000 kg/m3 and 1 ml = 1 cm3, calculate the amount of heat (in calories) released by water during this process. Take cwater = 1 cal/gK, cice = 0.5 cal/g and Lfusion = 80 cal/g.
Solution 5
Let's convert the clues into the required units first. We have
V = 200 ml = 200 cm3
t1 (water) = 20°C
t2 (ice) = -10°C
ρwater = 1000 kg/m3 = 1 g/cm3
cwater = 1 cal/gK
cice = 0.5 cal/g
Lfusion = 80 cal/g
Qtotal released = ?
First, we must work out the mass (in grams) of water, as it is needed during the solution. We have
= 1 g/cm 3 × 200 cm3
= 200 g
In this problem, water experiences three stages:
1) It cools down from 20°C to 0°C (the minimum temperature in which water can exist in liquid state). During this process, we must use the equation
= m × cw × (tmelting - t1(water) )
= 200 g × 1 cal/g∙°C × (0 - 20)°C
= -4000 cal
2) Then, water freezes without any change in temperature. During this process, we must use the equation
= -200 g × (-80)cal/g
= -1600 cal
3) Finally, the ice cols down from 0°C to -10°C. During this process, we must use the equation
= m × cice × (t2 (ice) -tmelting )
= 200 g × 1 cal/g × °C × (-10 - 0)°C
= -2000 cal
Therefore, we obtain for the total energy
= -4000 cal - 1600 cal - 2000 cal
= -7600 cal
This means the water loses 7600 cal of heat energy during this process.
You have reached the end of Physics lesson 13.3.5 Latent Heat. Specific Latent Heat of Fusion and Vaporization. There are 5 lessons in this physics tutorial covering Absorption of Heat, you can access all the lessons from this tutorial below.
More Absorption of Heat Lessons and Learning Resources
Whats next?
Enjoy the "Latent Heat. Specific Latent Heat of Fusion and Vaporization" physics lesson? People who liked the "Absorption of Heat lesson found the following resources useful:
- Latent Feedback. Helps other - Leave a rating for this latent (see below)
- Thermodynamics Physics tutorial: Absorption of Heat. Read the Absorption of Heat physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Absorption of Heat. Print the notes so you can revise the key points covered in the physics tutorial for Absorption of Heat
- Thermodynamics Practice Questions: Absorption of Heat. Test and improve your knowledge of Absorption of Heat with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Calorimetry (Heat Transfer)
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Absorption of Heat" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator