Menu
Physics Lesson 20.3.3 - Beta Decay
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Beta Decay, this is the third lesson of our suite of physics lessons covering the topic of Radioactivity and Half-Life, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Beta Decay
In the experiment of Becquerel, there were some traces on the film plate in the opposite direction to those produced by alpha particles. This means such traces are produced by negatively charged particles, as alpha particles are positive (they are helium nuclei). The name beta (β) particles was given to those moving in opposite direction to alpha ones and the naturally occurring decay process of such particles was called beta decay. Later on, it was discovered that such particles are nothing more but electrons.
Many years later, a new elementary particle known as positron, which has the same mass as electron but is positively charge, it was observed that there are two types of beta decay: beta minus (β-) and beta plus (β + ).
Beta decay occurs when there is excess of any particle (neutron or proton) in the atomic nucleus a neutron or a proton. As a result, any of extra particles splits in two parts. Thus, in beta minus decay, a neutron splits into one proton and one electron according to the relation:
while in a beta plus decay, a proton splits into one neutron and one positron according to the relation:
where e+ stands for the positron.
Since all splitting processes involve release or absorption of energy (that is, a third element in the right side of decay equation), it was evident that in the above reactions something is missing. This was also proven from experiments (energy and momentum are not conserved if we don't consider a third particle in the above equations). Obviously, such particle must be also elementary, as it is not proton, neutron or electron but something else. From experiments, this new elementary particle involved in the beta decay process was identified. In fact, there are two new elementary particles involved in a beta decay (given that there are two types of beta decay). One is called antineutrino (ν ) and it is involved in the beta minus decay, i.e.
and the other one (involved in beta plus decay) is called neutrino (ν). Thus, the equation of beta plus decay becomes
Antineutrino and neutrino do not contain any electric charge; they are involved in the process only for the energy conservation purpose. The discovery of these two elementary particles is attributed to Wolfgang Pauli - a German scientist who was the first to understand the need for a new particle to make the conservation laws (of energy and impulse) applicable in beta decay equations.
Both types of beta decay reactions occur for the purpose to make the daughter nucleus be closer to the stability zone, as shown in the graph below.
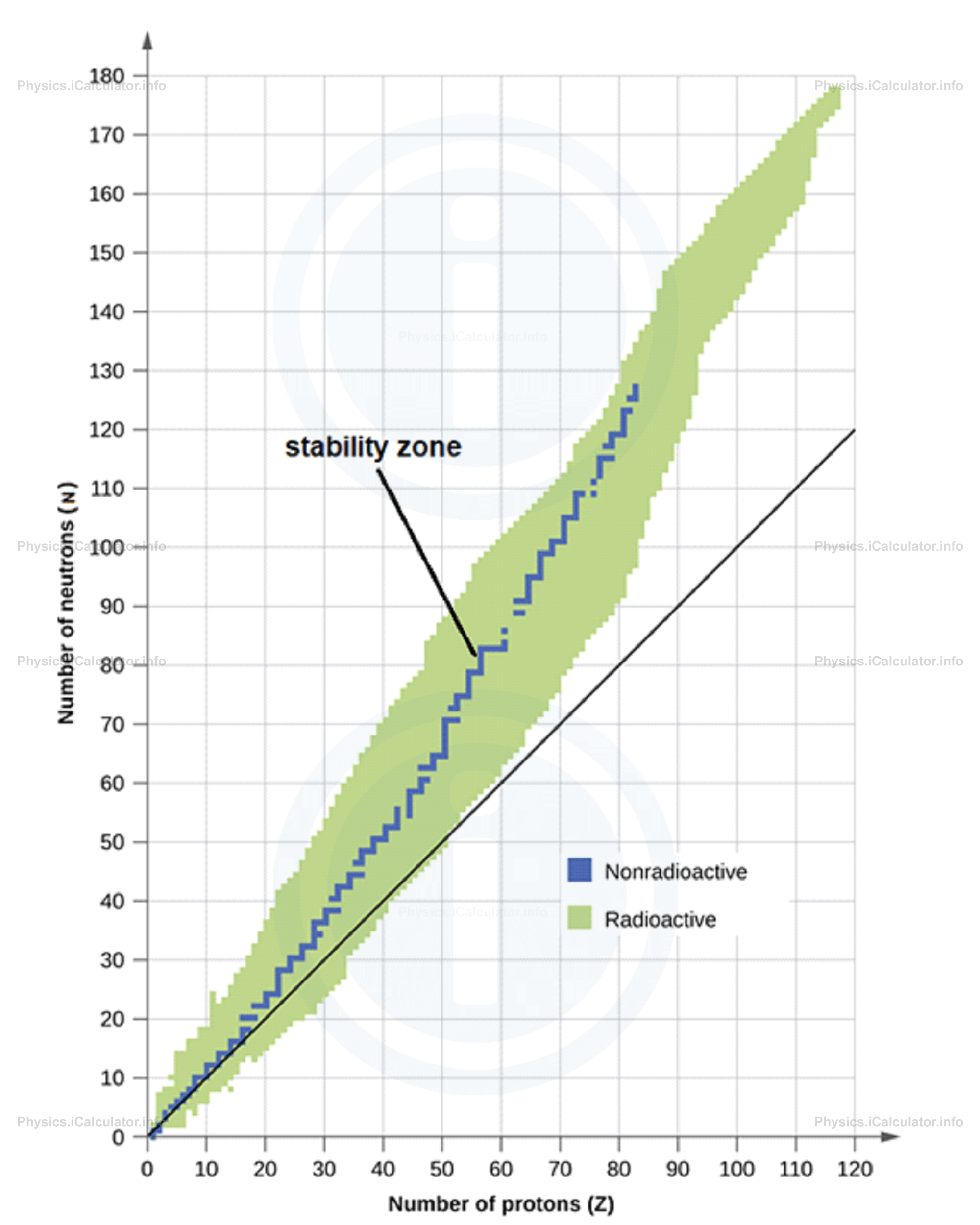
Neutrino and antineutrino (referred to by the common name "leptons") are particles that do not naturally exist in the nucleus; they are created only in the moment at which the beta decay is taking place. Both reactions of beta decay occur in such a way that the daughter particle moves closer to the stability zone. Let's consider the hydrogen nucleus to illustrate this fact. This nucleus contains only one proton and nothing else. Applying the equation of beta plus decay, it results that a proton splits into one neutron and one positron (including one neutrino as well). Hence, based on this approach a hydrogen nucleus cannot exist. However, the existence of hydrogen nuclei is matter of fact. This is because an isolated proton is a stable structure and it does not split in two parts. Beta decay occurs only in heavy nuclei that have a large number of protons and this process occurs for the only purpose to produce more stable structures (i.e. to obtain new nuclei with less difference between the number of protons and neutrons).
It is obvious that since the number of protons in the nuclei during a beta decay changes (it increases by 1 in β- and decreases by 1 in β + decay), a new chemical element is obtained, as the atomic number Z (which indicates the number of protons in an element) changes. On the other hand, the atomic mass number A remains the same in both types of beta decay as the number of nucleons does not change. We simply have a proton-to-neutron or neutron-to-proton conversion. The general formula of elements involved in both types of beta decays is
for beta minus decay and
for beta plus decay.
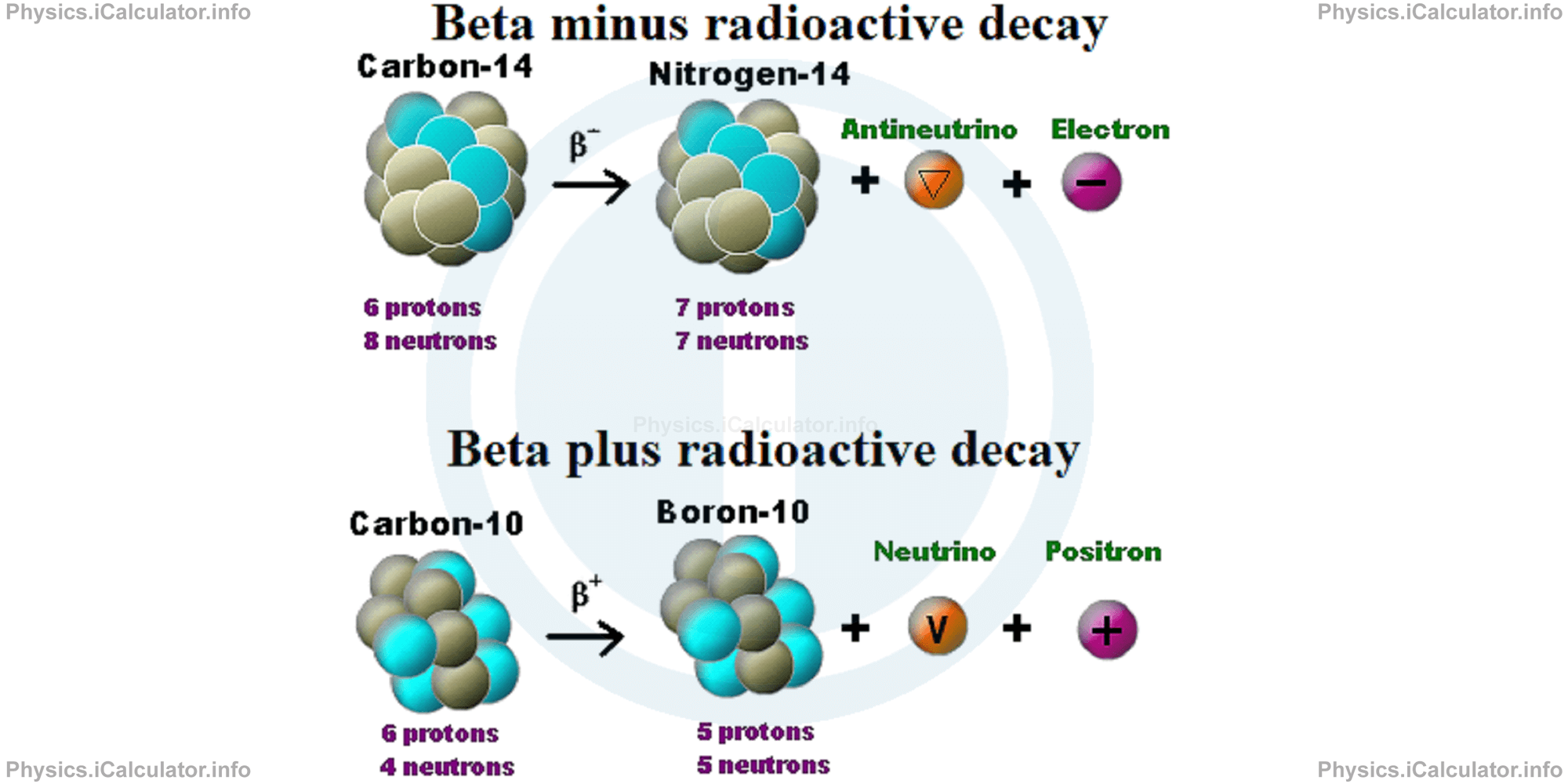
For example, a Carbon-14 nucleus (Carbon-14 has 6 protons and 8 neutrons in the nucleus, that is 6 + 8 = 14 nucleons in total) turns into a Nitrogen-14 nucleus after a beta minus decay (Nitrogen-14 contain 7 protons and 7 neutrons in the nucleus).
On the other hand, a Carbon-11 nucleus (Carbon-11 contains 6 protons and 5 neutrons in the nucleus) can turn into Boron-11 (Boron-11 contains 5 protons and 6 neutrons in the nucleus) by means of a beta plus decay, that is when a proton converts to neutron.
From the above examples, we reach the following conclusion:
Beta minus decay occurs when the parent nucleus has excess of neutrons, while beta plus decay occurs when the parent nucleus has excess of protons. In both cases, more stable nuclei are obtained after the beta decay process.
Tip to make it easy to remember the name of beta decay involved: Always look at whether an electron or positron is emitted during the decay. If there is an electron emitted, then we have a beta minus decay as electron is negative. On the other hand, since positron is positive, there is a beta plus decay when a positron is emitted.
The following figure shows how the two types of beta decay take place.
Example 2
What type of radioactive decay takes place when Fluoroine-18 converts to Oxygen-18?
Solution 2
Since the number of nucleons does not change, we have a beta decay involved (alpha decay makes the number of nucleons decrease by 4 as a helium nucleus is emitted during this process).
Now, we have to find what type of beta decay occurs in this case. From periodic table, we see that Fluorine (F) contains 9 protons (Z = 9) while Oxygen (O) contains 8 protons (Z = 8) in their respective nuclei. This means a proton is converted to neutron when Fluorine-18 turns to Oxygen-18. This is an example of beta plus decay, i.e. a positron is involved in the process (and a neutrino as well). The reaction that takes place in this process therefore is
We can write the symbol (01e ) instead of (e+ ) to indicate a positron. This is because positron is produced during a beta plus decay when the atomic number (number of protons therefore) decreases by 1 (one proton turns into one neutron and one positron), so, we want to show this fact on the right side of reaction. Likewise, we can use the symbol (0-1e ) to indicate and electron produced during a beta minus decay as this electron is obtained when the atomic number increases by 1, i.e. when one neutron turns into one proton and one electron. Thus, for example, instead of writing
we can write
This notation is easier to understand as you can do the operation with subscripts and superscripts (indicating protons and nucleons respectively) in both sides to see whether the law of mass conservation is applied correctly (here we have 9 = 8 + 1 and 18 = 18 + 0).
In such a notation, we don't write the neutrino or antineutrino as they do not contain any charge or particle, but their presence is implied.
During beta decay processes, energy and momentum are both conserved. Since in energy sharing process after the decay three particles are involved (daughter nucleus, beta particle and neutrino/antineutrino), but we have only two laws of conservation (of energy and momentum), then the energy sharing is not well-determined. Likewise, the binding energy of daughter nucleus varies based on the specific situation. This energy is the sum of energies of beta particle, daughter nucleus and neutrino/antineutrino. Given that a number of factors are involved in this energy sharing, the amount of energy each particle shares is not determined, despite the laws of conservation are applied in each case. As a result, the beta particle produced has not a determined energy after the decay.
Example 3
Calculate the energy obtained during the β decay in which Carbon-14 turns into Nitrogen-14. Take the mass of Carbon-14 as 14.003242 u and that of Nitrogen-14 as 14.003074 u.
Solution 3
This radioactive decay is written as
The C-14 atom is neutral; this means 6 electrons revolve around its nucleus. When it turn into N-14, the new atom is not neutral anymore, it is positively charged as it still contains 6 electrons but now it has 7 protons. Actually, the mass of daughter nucleus (N-14) plus 6 electrons revolving around it plus the mass of electron radiated gives the mass of neutral N-14 atom. Giving that the mass of neutral N-14 atom is 14.003074 u, the change in mass (mass defect) in this case is
= 14.003242 u - 14.003074
= 0.000168 u
= 1.68 × 10-4 u
When converted to kg, this value becomes
= 2.7897 × 10-31 kg
Hence, the energy produced during this beta (minus) decay process is
= (2.7897 × 10-31 kg) ∙ (3 × 108 )2
= 2.51 × 10-14 J
This energy is carried by beta particles in the form of kinetic energy, as the daughter particle is much heavier that the beta particle produced (which is an electron). As a result, the daughter particle (N-14) is assumed as non-moveable and therefore, all the energy obtained during the decay goes to the electron in the form of kinetic energy.
You have reached the end of Physics lesson 20.3.3 Beta Decay. There are 5 lessons in this physics tutorial covering Radioactivity and Half-Life, you can access all the lessons from this tutorial below.
More Radioactivity and Half-Life Lessons and Learning Resources
Whats next?
Enjoy the "Beta Decay" physics lesson? People who liked the "Radioactivity and Half-Life lesson found the following resources useful:
- Beta Decay Feedback. Helps other - Leave a rating for this beta decay (see below)
- Nuclear Physics Physics tutorial: Radioactivity and Half-Life. Read the Radioactivity and Half-Life physics tutorial and build your physics knowledge of Nuclear Physics
- Nuclear Physics Revision Notes: Radioactivity and Half-Life. Print the notes so you can revise the key points covered in the physics tutorial for Radioactivity and Half-Life
- Nuclear Physics Practice Questions: Radioactivity and Half-Life. Test and improve your knowledge of Radioactivity and Half-Life with example questins and answers
- Check your calculations for Nuclear Physics questions with our excellent Nuclear Physics calculators which contain full equations and calculations clearly displayed line by line. See the Nuclear Physics Calculators by iCalculator™ below.
- Continuing learning nuclear physics - read our next physics tutorial: Nuclear Reactions
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Radioactivity and Half-Life" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.