Menu
Physics Lesson 20.3.2 - Alpha Decay
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Alpha Decay, this is the second lesson of our suite of physics lessons covering the topic of Radioactivity and Half-Life, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Alpha Decay
In alpha (α) decay or disintegration, a heavy (massive) nucleus emits a helium (42He) nucleus and another daughter nucleus. For example, any of uranium isotopes such as (23892U) may emit an alpha particle and thus become a thorium isotope (23490Th). The mathematical relation in alpha decay is
Alpha particles were given this name prior to discovering what kind of particles they represent. However, now we know that alpha particles are nothing more but helium nuclei. An alpha particle is a very stable structure (we have explained that hydrogen and helium are very stable materials; indeed the Sun is mainly composed by hydrogen and helium elements).
Alpha particles detach from their parent nuclei because during the attempt to reduce the repelling electric forces, alpha particles, which are formed inside the nucleus, may find themselves in the periphery of nucleus and gain enough kinetic energy to leave it without any interference from an external source of energy that is to overcome the nuclear binding force. Alpha decay is schematically shown in the figure below.
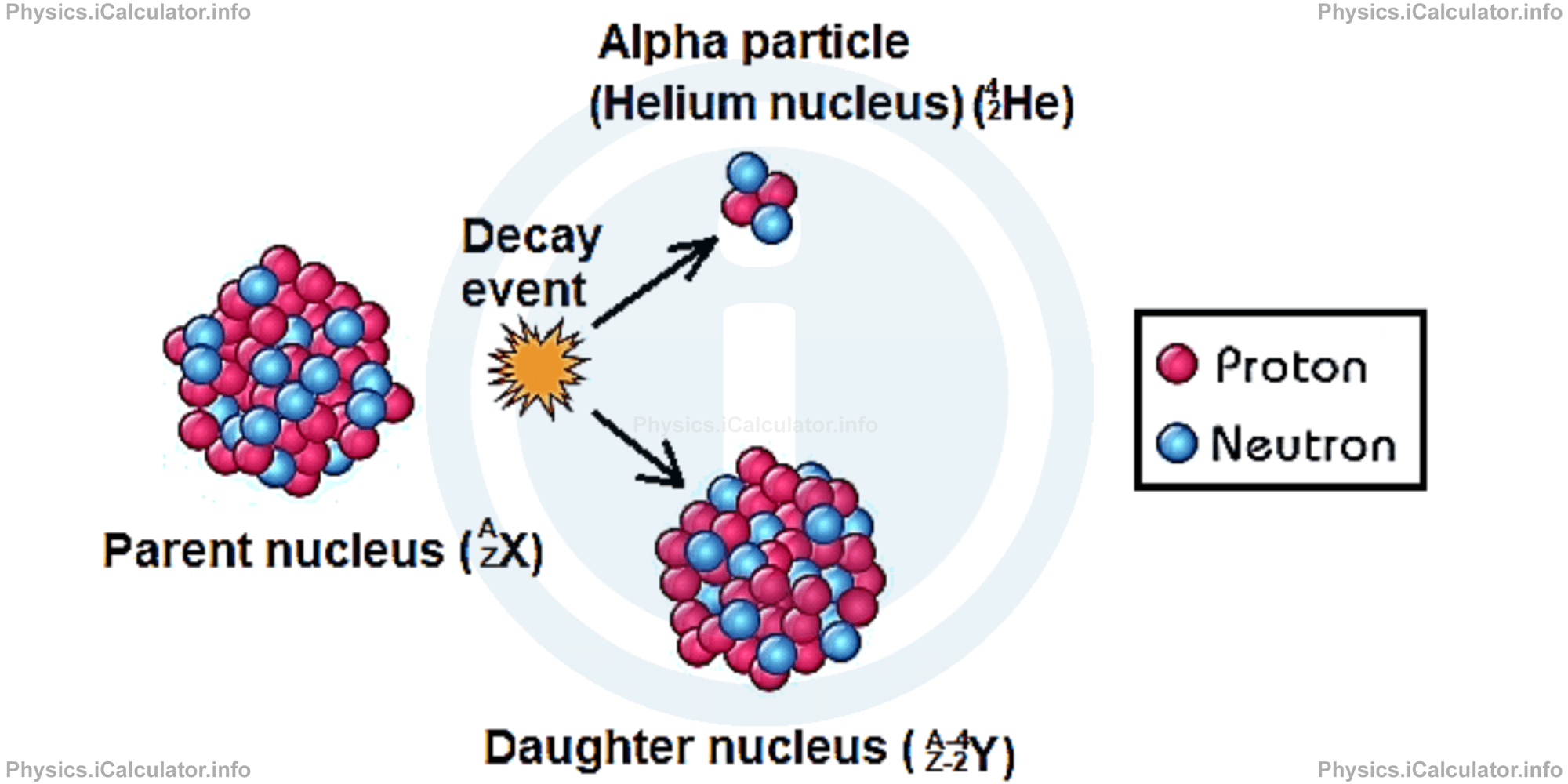
This process has a probabilistic nature; this means none of particles is favoured at start but everything depends on their actual arrangement inside the nucleus at a given instant. A probabilistic process always has a non-zero chance to occur, despite the conditions may be such that the event seems improbable. This occurs only in micro-world, not in real life. For example, the probability for an athlete to jump 10 m high without any aiding tool is zero as this exceeds the human physical capabilities but in micro-world nothing is improbable. A particle may overcome obstacles that may seem impossible - this is known as the "tunnel effect".
When an alpha particle leaves the original nucleus, a more stable nucleus is formed. We have explained in the previous article that the proton-neutron ratio (or vice-versa) is an indicator on the nuclei stability. For example, in the alpha decay process shown below
the proton-neutron ratio of "parent" nucleus (Polonium, Po) is
and the proton-neutron ratio of "daughter" nucleus (Lead, Pb) is
Despite the change in ratio is small, it is sufficient to make the daughter nucleus shift from radioactive to stable region of the N vs Z graph given in the previous article. When an alpha decay takes place, the electric charge in the daughter nucleus bemomes smaller than in the parent nucleus. As a result, the binding energy in daughter nucleus is smaller too. This results in a more stable nucleus. Moreover, the nuclear mass also decreases, bringing a decrease in the stored energy in the daughter nucleus (recall the mass-energy equivalence).
From the law of energy conservation, it is obvious that this difference in energy between parent and daughter nuclei convers into kinetic energy of the daughter particle and helium nucleus (recall the law of conservation of momentum in explosions). Therefore, we may use the law of conservation of momentum to determine how fast the daughter nucleus and helium nucleus will move after an alpha-decay process does occur.
Example 1
Calculate the energy released when a Seaborgium (263106Sg) nucleus experiences an alpha decay. Refer to the previous article for any useful information. Seaborgium nucleus is considered at rest and the two new particles move in opposite directions after the alpha decay takes place.
Solution 1
First, it is useful to provide an overview of the situation. Since all particles possess some rest energy in the form of mass, which we can find through the mass-energy equivalence method, we can then find the change in energy by comparing them. This change in energy (which is the binding energy of daughter and helium nucleus when they were in the parent nucleus) represents the sum of kinetic energies of the new particles produced due to alpha decay, which corresponds to the energy released by the Seaborgium nucleus during this process.
Giving that the decay process that occurs in this reaction is
and giving that atomic masses of these three materials are 266 u, 261 u and 4.003 u respectively, we obtain for the mass defect of this process:
= 266 u - (261 u + 4 u)
= 1u
Since this value corresponds to 1.66054 × 10-27 kg, we obtain for the binding energy of parent nucleus:
= (1.66054 × 10-27 kg) ∙ (3 × 108 m/s2 )2
= 1.49449×10-10 J
This energy corresponds to energy released during the alpha decay; it is in the form of kinetic energy.
You have reached the end of Physics lesson 20.3.2 Alpha Decay. There are 5 lessons in this physics tutorial covering Radioactivity and Half-Life, you can access all the lessons from this tutorial below.
More Radioactivity and Half-Life Lessons and Learning Resources
Whats next?
Enjoy the "Alpha Decay" physics lesson? People who liked the "Radioactivity and Half-Life lesson found the following resources useful:
- Alpha Decay Feedback. Helps other - Leave a rating for this alpha decay (see below)
- Nuclear Physics Physics tutorial: Radioactivity and Half-Life. Read the Radioactivity and Half-Life physics tutorial and build your physics knowledge of Nuclear Physics
- Nuclear Physics Revision Notes: Radioactivity and Half-Life. Print the notes so you can revise the key points covered in the physics tutorial for Radioactivity and Half-Life
- Nuclear Physics Practice Questions: Radioactivity and Half-Life. Test and improve your knowledge of Radioactivity and Half-Life with example questins and answers
- Check your calculations for Nuclear Physics questions with our excellent Nuclear Physics calculators which contain full equations and calculations clearly displayed line by line. See the Nuclear Physics Calculators by iCalculator™ below.
- Continuing learning nuclear physics - read our next physics tutorial: Nuclear Reactions
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Radioactivity and Half-Life" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.