Menu
Physics Lesson 9.7.2 - Surface Tension
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Surface Tension, this is the second lesson of our suite of physics lessons covering the topic of Adhesive and Cohesive Forces. Surface Tension and Capillarity, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Surface Tension
As stated above, molecules in a liquid pull each other by means of cohesive forces. There is an equilibrium between these forces inside the liquid because any molecule inside the liquid is pulled in all direction by other similar molecules, and therefore the resultant of all cohesive forces acting on that molecule is zero.
Only on the molecules that are on the upper layer of the liquid the resultant of cohesive forces is not zero, as they do not have other molecules above to pull them. Molecules of the upper layer are pulled by other molecules below and as a result, a non-zero resultant force in the inward direction is produced.
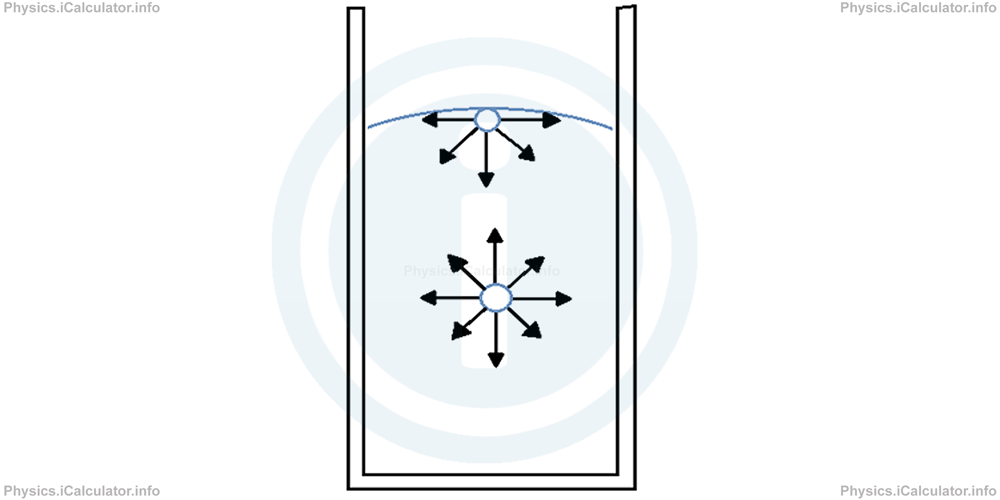
This force is known as the force of surface tension, F, and it causes liquids to shrink and take a spherical shape (as in water droplets we discussed earlier).
Do not confuse surface tension and surface tension force. Surface tension, γ is an intrinsic property of the liquid itself (like density, freezing temperature, colour, texture, etc.) It depends neither on environmental conditions nor on the material structure but only on the surface tension force F and the length L of the part of liquid surface in contact with the surrounding substance. The equation of surface tension is:
Obviously, the unit of surface tension γ is [N/m]. From the above formula, we obtain for the surface tension force,
Example 1
The surface tension of water in 20°C is 72 8 mN/m (milli Newtons per metre). What is the surface tension force exerted by water if it is poured inside a narrow tube of diameter 8 mm?
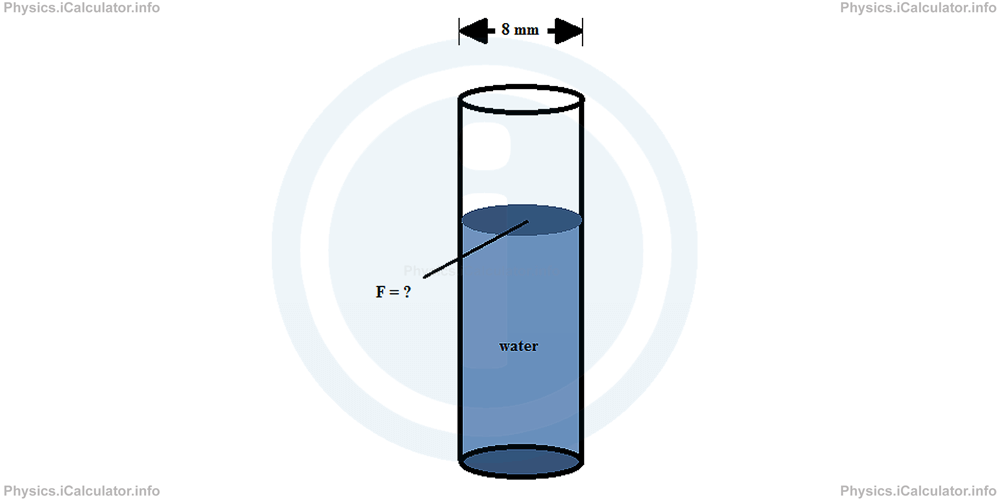
Solution 1
From theory, we know that surface tension force is calculated by the formula
where γ is the surface tension (here γwater = 72.8 mN/m = 0.0728 N/m) and L is the length of the part of water surface in contact with the tube.
Since the tube is cylindrical-shaped, the above length L represents the circumference of cylinder's base, i.e.
= 2 × π × d/2
= π × d
where d is the diameter of the tube (d = 8 mm = 0.008 m).
Hence, we obtain for the surface tension force F:
= 0.0728 N/m × 3.14 × 0.008 m
= 0.0018 N
As you see, the force of surface tension is very small. However, when the surface increases this force helps heavy objects such as ships to float more easily on water due to the compactness that water molecules produce during their shrinkage.
You have reached the end of Physics lesson 9.7.2 Surface Tension. There are 3 lessons in this physics tutorial covering Adhesive and Cohesive Forces. Surface Tension and Capillarity, you can access all the lessons from this tutorial below.
More Adhesive and Cohesive Forces. Surface Tension and Capillarity Lessons and Learning Resources
Whats next?
Enjoy the "Surface Tension" physics lesson? People who liked the "Adhesive and Cohesive Forces. Surface Tension and Capillarity lesson found the following resources useful:
- Surface Tension Feedback. Helps other - Leave a rating for this surface tension (see below)
- Density and Pressure Physics tutorial: Adhesive and Cohesive Forces. Surface Tension and Capillarity. Read the Adhesive and Cohesive Forces. Surface Tension and Capillarity physics tutorial and build your physics knowledge of Density and Pressure
- Density and Pressure Revision Notes: Adhesive and Cohesive Forces. Surface Tension and Capillarity. Print the notes so you can revise the key points covered in the physics tutorial for Adhesive and Cohesive Forces. Surface Tension and Capillarity
- Density and Pressure Practice Questions: Adhesive and Cohesive Forces. Surface Tension and Capillarity. Test and improve your knowledge of Adhesive and Cohesive Forces. Surface Tension and Capillarity with example questins and answers
- Check your calculations for Density and Pressure questions with our excellent Density and Pressure calculators which contain full equations and calculations clearly displayed line by line. See the Density and Pressure Calculators by iCalculator™ below.
- Continuing learning density and pressure - read our next physics tutorial: Fluids. Density of Fluids
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Adhesive and Cohesive Forces. Surface Tension and Capillarity" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.