Menu
Physics Lesson 13.8.4 - Degrees of Freedom and Molar Specific Heats
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Degrees of Freedom and Molar Specific Heats, this is the fourth lesson of our suite of physics lessons covering the topic of Molar Specific Heats and Degrees of Freedom, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Degrees of Freedom and Molar Specific Heats
With degrees of freedom, we understand independent ways of a particle's motion. For example, a train moving on a railway has only one degree of freedom as it can move only according one direction - the direction determined by the tracks.
A translational motion can be a combination of motion in three linear directions, which we usually call X, Y and Z according the names of the three well-known perpendicular axes we have already seen in previous tutorials.
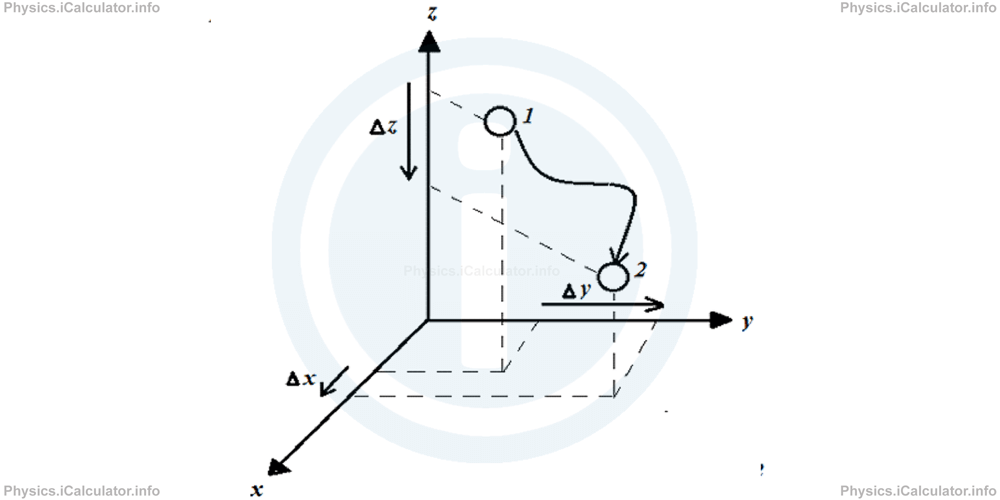
All atoms in monoatomic gases are assumed to move only in a translational way as even if they spin around themselves, this is not observable and relevant. Indeed, when you watch a football match, you don't care about the spinning effect of ball but only about its translational motion. This is why the internal energy of monoatomic gases is equal to
The number 3 is because there are three degrees of freedom in translational motion. The division by 2 is because every individual direction includes two sub-directions in itself. (left-right, back-forth, up-down).
In diatomic (two-atomic) molecules, there is a spinning effect produced which causes a double direction of rotation.
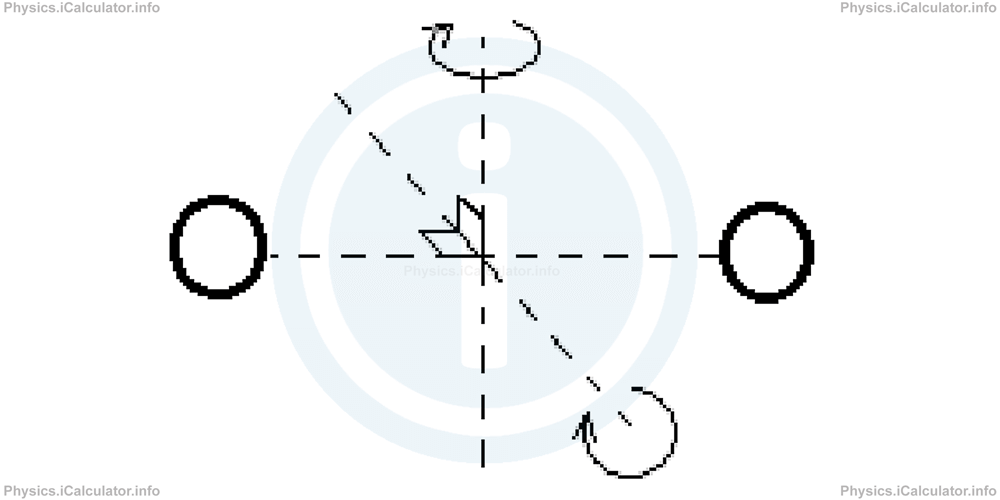
Therefore, in total we have 5 degrees of freedom for the internal energy, i.e.
If molecules are three or more atomic (polyatomic), there is another spinning direction added because two points form a line (one direction) while three point form a plane (two directions). Therefore, the total internal energy becomes
= 6/2 × n × R × T
= 3 × n × R × T
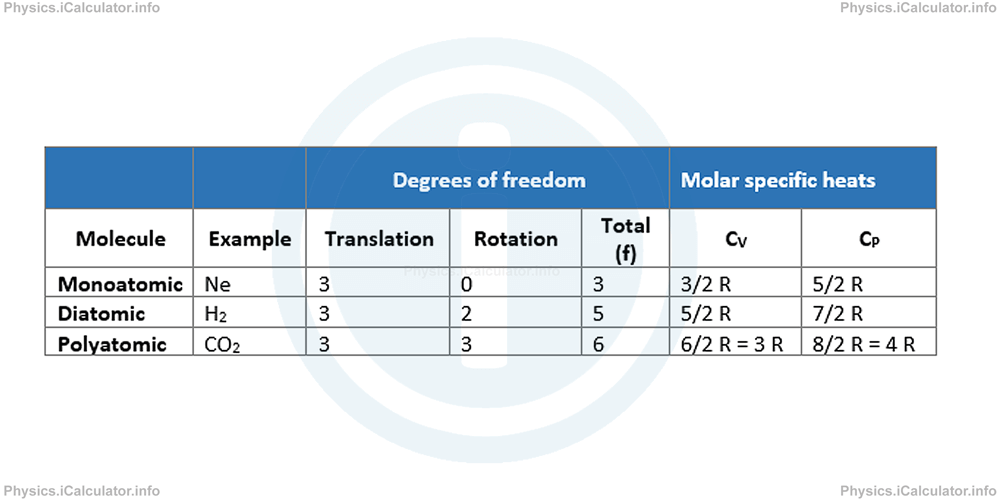
The table below shows the relationship between molar specific heats and degrees of freedom in ideal gases.
Based on the above analysis, James Clerk Maxwell formulated the theorem of the equipartition of energy, which says:
Every molecule has a certain number of degrees of freedom, which represent independent ways of storing energy. A portion of energy associated with a single degree of freedom is given by the expression
U = 1/2 kT (for a single molecule) or U = 1/2 RT (for a mole)
Thus, we can obtain a general form of the relationship between the number of degrees of freedom f and the molar specific heat at constant volume CV for an ideal gas in terms of the ideal gas constant R:
= f × 8.31/2 J/mol × K
= 4.16 × f J/mol × K
There is also a vibrational motion caused by the oscillations of diatomic or polyatomic molecules, whose behaviour is described through quantum theory, but which goes beyond the scope of this tutorial. It required higher amounts of energy to take place then the other two types of motion explained here. Therefore, we neglect this kind of motion when energy stored in molecules is relatively small.
You have reached the end of Physics lesson 13.8.4 Degrees of Freedom and Molar Specific Heats. There are 4 lessons in this physics tutorial covering Molar Specific Heats and Degrees of Freedom, you can access all the lessons from this tutorial below.
More Molar Specific Heats and Degrees of Freedom Lessons and Learning Resources
Whats next?
Enjoy the "Degrees of Freedom and Molar Specific Heats" physics lesson? People who liked the "Molar Specific Heats and Degrees of Freedom lesson found the following resources useful:
- Knowledge Feedback. Helps other - Leave a rating for this knowledge (see below)
- Thermodynamics Physics tutorial: Molar Specific Heats and Degrees of Freedom. Read the Molar Specific Heats and Degrees of Freedom physics tutorial and build your physics knowledge of Thermodynamics
- Thermodynamics Revision Notes: Molar Specific Heats and Degrees of Freedom. Print the notes so you can revise the key points covered in the physics tutorial for Molar Specific Heats and Degrees of Freedom
- Thermodynamics Practice Questions: Molar Specific Heats and Degrees of Freedom. Test and improve your knowledge of Molar Specific Heats and Degrees of Freedom with example questins and answers
- Check your calculations for Thermodynamics questions with our excellent Thermodynamics calculators which contain full equations and calculations clearly displayed line by line. See the Thermodynamics Calculators by iCalculator™ below.
- Continuing learning thermodynamics - read our next physics tutorial: Gas Laws
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Molar Specific Heats and Degrees of Freedom" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.
Thermodynamics Calculators by iCalculator™
- Carnot Engine Efficiency Calculator
- Entropy Calculator
- Gas Laws Calculator
- Molecular Mean Free Path Calculator
- Translational Kinetic Energy Of Gas Calculator
- Root Mean Square Speed Calculator
- Ideal Gas Law Calculator
- Change In The Gas Internal Energy Calculator
- Radiative Heat Transfer Calculator
- Evaporative Heat Transfer Calculator
- Convective Heat Transfer Calculator
- Conductive Heat Transfer Calculator
- Final Temperature Of Mixture Calculator
- Heat Absorbed Or Released Calculator
- Thermal Expansion Calculator
- Temperature Calculator