Menu
Physics Lesson 21.1.1 - Background and Introduction to Quantum Numbers and Orbitals
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on Background and Introduction to Quantum Numbers and Orbitals, this is the first lesson of our suite of physics lessons covering the topic of Elementary Particles, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
Background and Introduction to Quantum Numbers and Orbitals
As we discussed in Section 20 (Nuclear Physics), atoms are composed by a nucleus at centre that contains protons and neutrons, as well as electrons that revolve around the nucleus in determined orbits (layers or shells). However, when studying the atom more in detail, we see that a shell may contain a number of subshells, and so on. The more closely we observe the atom, the more specific features we discover in it.
a. Quantum numbers
Quantum numbers represent a method used to define the trajectory and movement of an electron within an atom. There are four quantum numbers for every electron in an atom, which are combined to give a unique configuration. This is like describing a person physically through a number of attributes which provide information to other people who don't know him (for example tall, blonde, slim and smiley). Quantum number in itself is a value used to describe the energy available in atoms or ions. Quantum numbers are nothing more but different shapes of orbitals drawn, depending on the possibility of finding electrons around the nucleus in the space enclosed by such shapes. The four quantum numbers are:
- n - the principal quantum number that expresses the energy levels (atomic orbitals). The principal quantum number n is always an integer. Additionally, it is equivalent to the number of electron shells. Hence, its value is at least one and higher. Principal quantum number is never zero or negative (n = 1, 2, 3, 4 ) as any atom has at least one electronic shell. Only four principal quantum numbers are in use so far for known elements, despite theoretically they can be more than four). This is because the number of chemical elements is finite (118 to this date).
- ℓ - the azimuthal or angular momentum quantum number that describes the subshell. The angular momentum quantum number is also an integer, which represents the value of an electron's orbital. Hence, ℓ is either greater than or equal to zero, and lower or equivalent to n -1 (ℓ = 0, 1, 2, , n - 1).
- mℓ (or simply m) - the magnetic quantum number that expresses the orbital of subshells, that is the mathematical functions often employed in order to determine the probability of finding an electron (belonging to an atom) in a specific region around the nucleus of the atom. The magnetic quantum number symbolizes the orientation of the orbital. The integer values of magnetic quantum number are ranging from -ℓ to +ℓ. Thus, for p orbital, where ℓ = 1, the magnetic quantum number m can have values of -1, 0, 1.
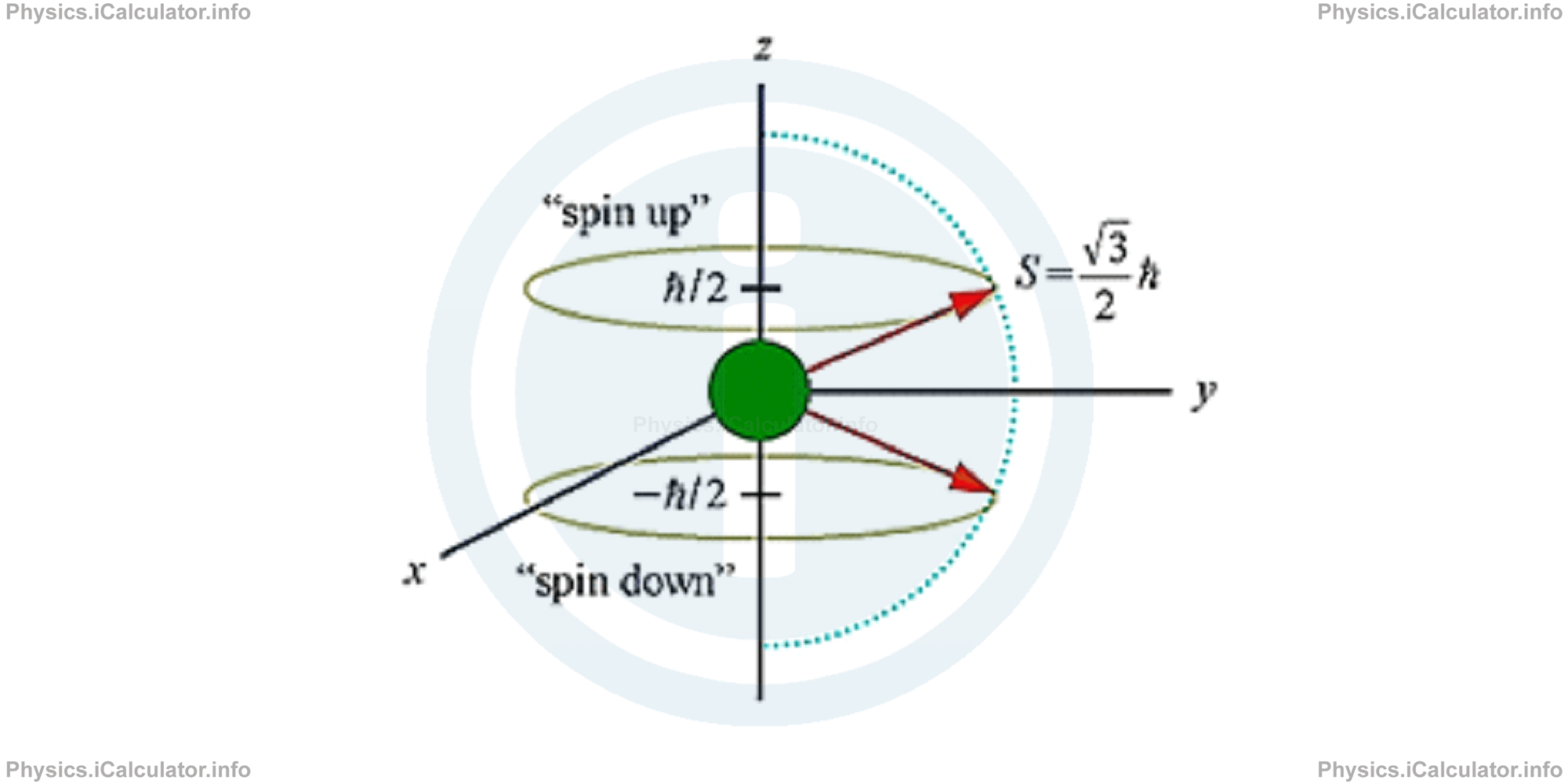
- ms (or simply s) - the spin quantum number expressing the spin, that is one of two types of angular momentum in quantum mechanics (the other is orbital angular momentum). The spin quantum number has a half-integer value, which is either - 1/2, known as 'spin down' or + 1/2 called 'spin up'. In practice, it describes the intrinsic angular momentum or 'spin' of an electron within an orbital, as the word itself suggests. Moreover, it provides a projection of the spin angular momentum (s) along a particular axis.
b. Atomic orbitals
The "orbit" is defined as the definite path of an electron that moves around the nucleus in an atom. This is similar to a planet which moves around the sun. Atomic orbitals on the other hand, are the space or region around the nucleus where the electron are calculated to be present. So orbits and orbitals have totally different meanings and it is important you remember that key difference..
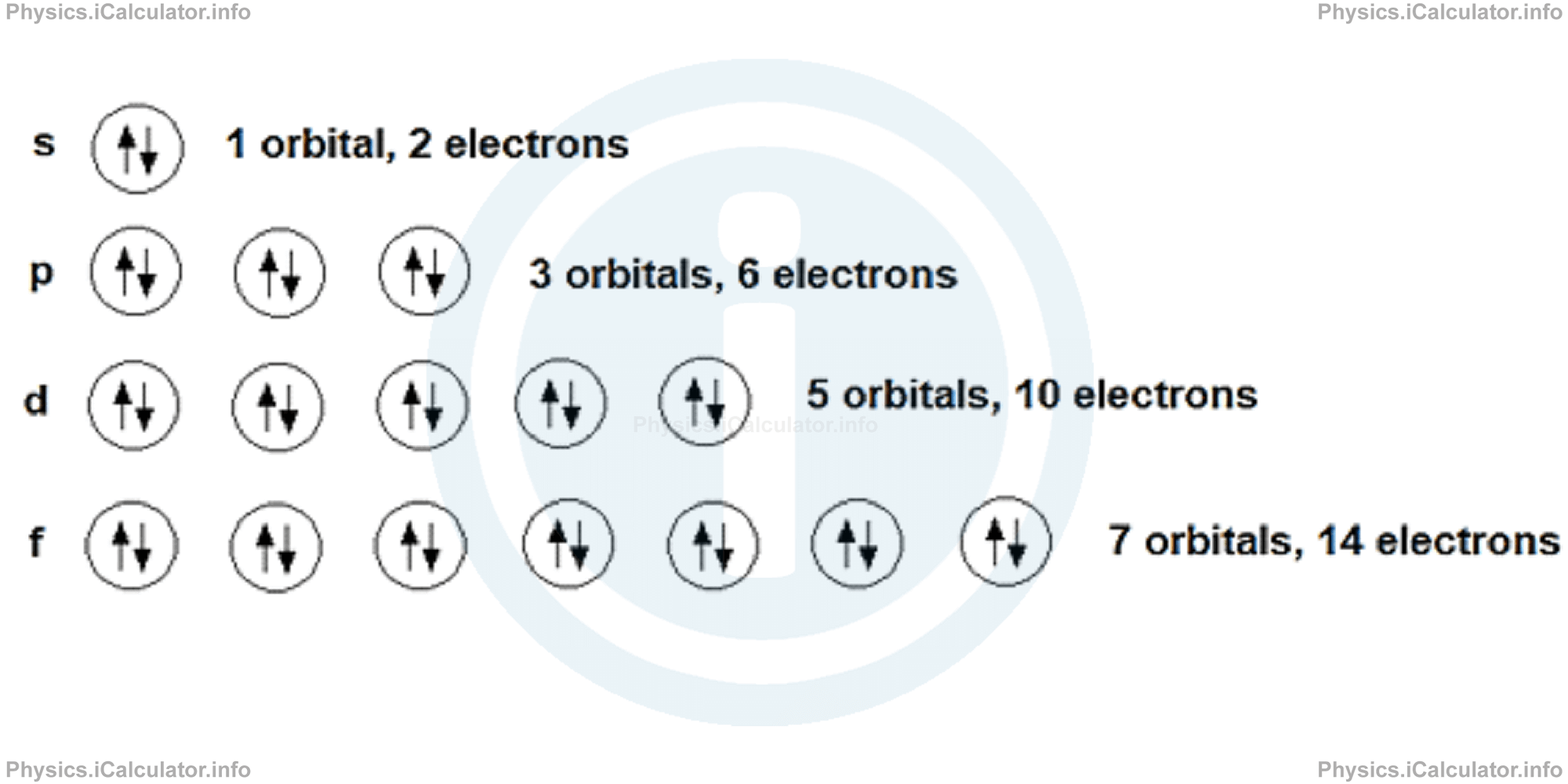
We understand from lessons in chemistry that the four atomic orbitals in use are s, p, d and f where s can hold 2 electrons at maximum, p can hold 8 electrons, d can hold 18 electrons and f can hold 32 electrons (that is 2n2 where n is the principal quantum number).
Let's describe more in detail the four orbitals and their orientation.
s-orbitals
s-orbitals represent solid spherical shapes around the nucleus. When the principal quantum number n = 1 and azimuthal quantum number ℓ = 0, that is 1s orbital which is closest to the nucleus. When n = 2 and ℓ = 0, (i.e 2s orbital) there is an orbital which contains one node. When n = 3 and ℓ = 0, (i.e. 3s), we have an orbital which contains two nodes. The pictorial representation of these orbitals is shown below:
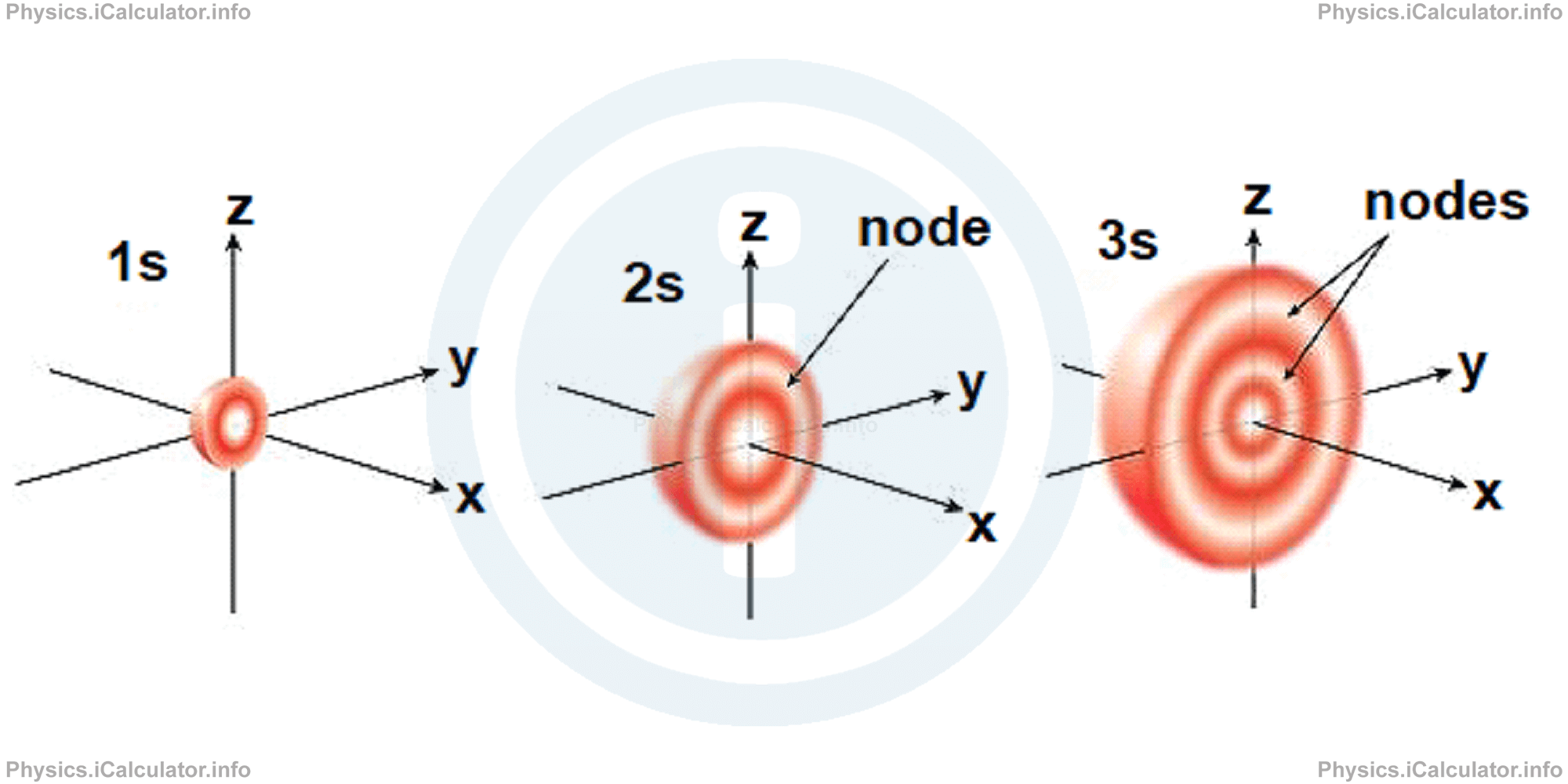
Hence, we can say that s-orbitals have always a spherical shape, regardless of the principal quantum number, size and the number of nodes they contain.
p-orbitals
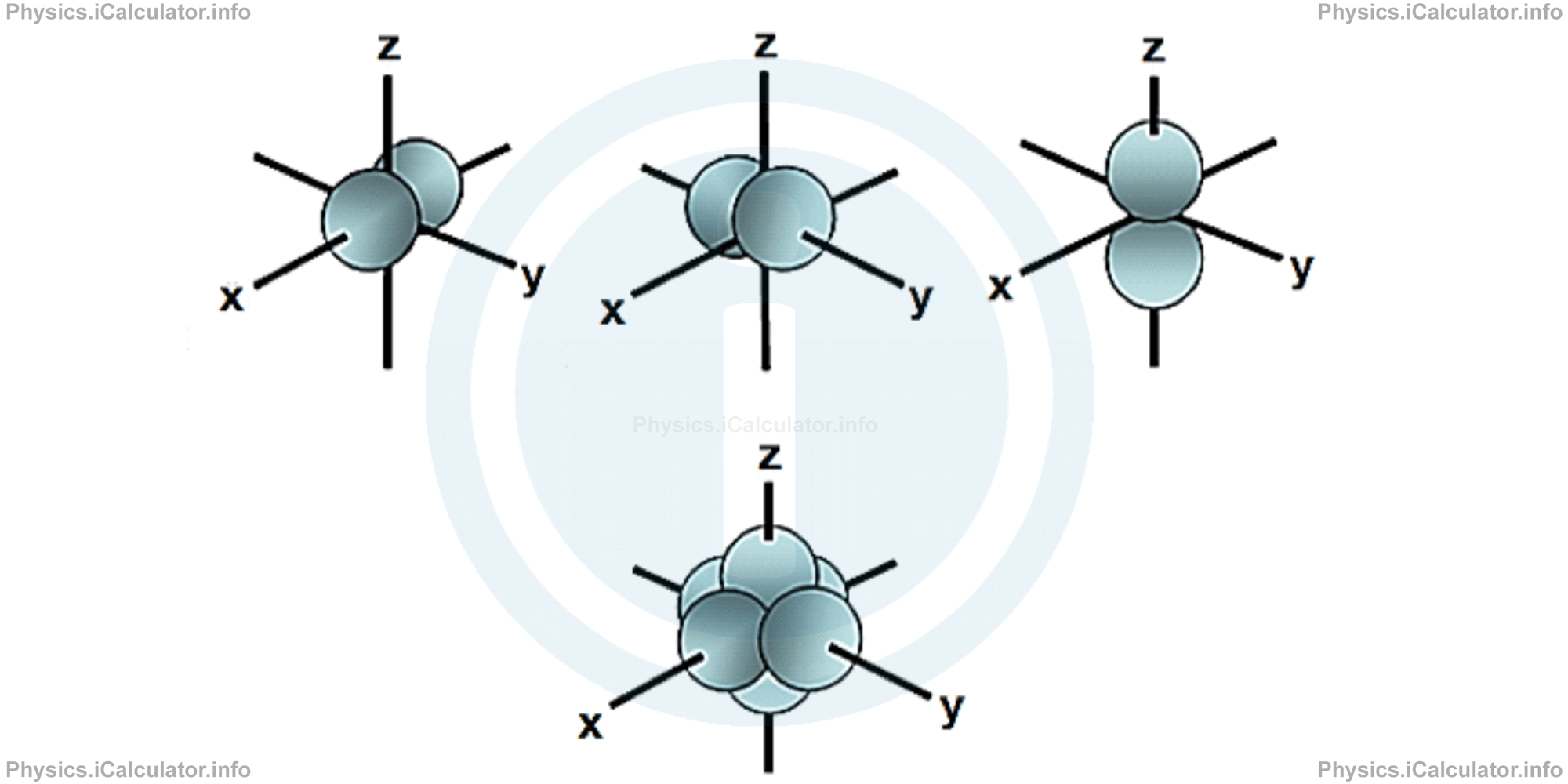
p-orbitals are dumb-bell shapes containing two lobes, like two identical balloons tied together. The two lobes are apart from each other along the axial line. When n = 1, there are no p-orbitals but only a s-orbital. When n = 2 and ℓ = 1, the possible magnetic quantum numbers are m = -1, 0, +1. Thus, three dumb-bell shape p-orbitals are found pointing towards the three axes x, y and z, which are perpendicular to each other. These three orbitals are named as px , py and pz respectively. The nodal plane is the plane where it is not possible to find any electrons. The nodal planes of px , py , pz are yz , xz and xy respectively.
d-orbitals
d-orbitals have different shapes and these are only available when the principal quantum number is n = 3 or more. When n = 3, then ℓ = 2, so the magnetic quantum number m can take the following values: m = -2, -1, 0, +1 and +2. That means five d-orbitals are available in any atom. The directions, names and the shapes of these orbitals are as follows:
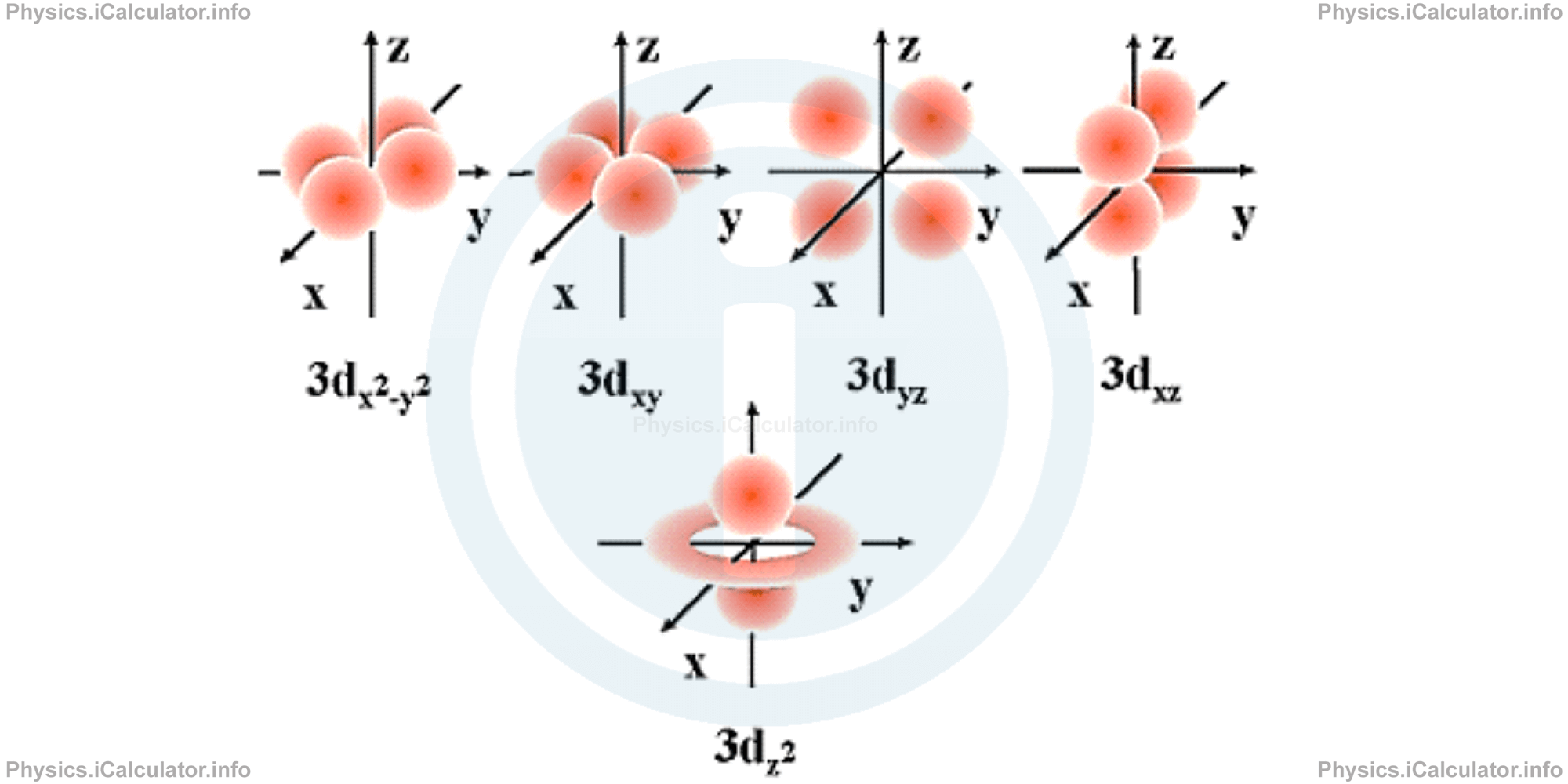
You don't have to remember all these configurations and symbols; they are just for illustration purpose as such things are extensively explained in chemistry courses.
f-orbitals
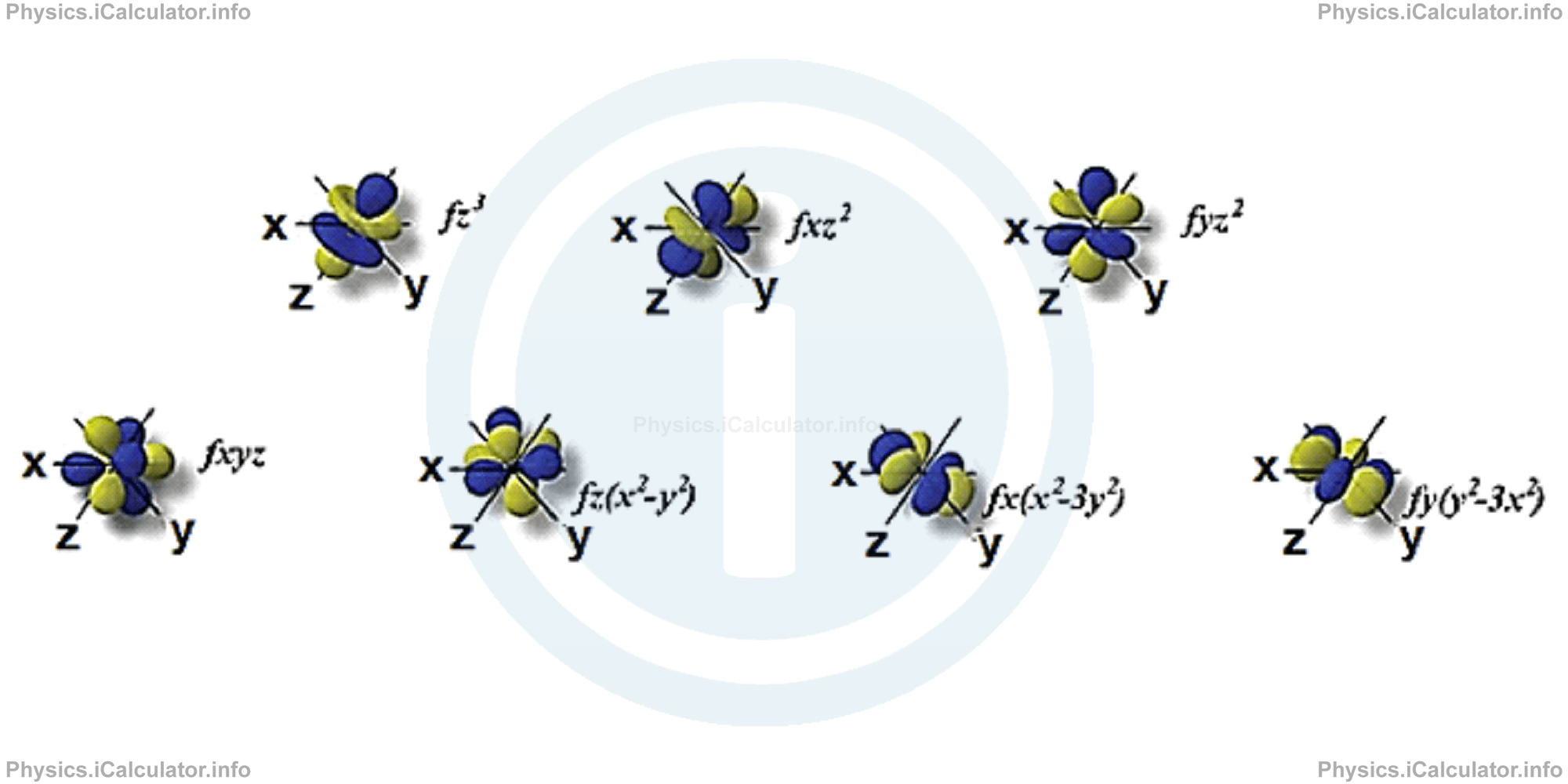
f-orbitals also have different shapes and they are only available when the principal quantum number is n = 4 or more. When n = 4, then ℓ = 3, so m = +3, +2, +1, 0, +1, +2 and +3. In this way, seven f-orbitals are available in an atom. The directions, names and the shapes of these orbitals are as follows:
c. Electrons distribution in orbitals
s-orbitals
The maximum number of electrons that each s-orbital can hold is two, regardless of the number of principal quantum number (n). Thus, we have 1s2, 2s2, 3s2 etc. The spin of these two electrons must be opposite, as stated earlier.
p-orbitals
Each p-orbital can possess at maximum two electrons which means six electrons in total; two electrons for each of three p-orbitals. We can write that either 2p6 or 2px2 2py2 2pz2. The spin of each of these orbitals must be opposite.
d and f-orbitals
The total number of electrons in d-orbitals and f-orbitals is 10 and 14 respectively. Again, two electrons at maximum can occupy each suborbital of these d or f-orbitals.
On the other hand, the number of orbitals is found through the formula
where ℓ is the azimuthal quantum number.
d. Spin quantum number
The spin of every two electrons, in each orbitals, will be always be in the opposite direction. Spin is represented schematically through vertical arrows, where each suborbital (orbital in a given direction) is represented through a square-shaped box. The following image shows the maximum electron distribution in each orbital.
The following figure shows what "spin up" and "spin down" mean in the trajectory of an electron.
There is an important principle (known as Pauli's Exclusion Principle) to bring in mind when dealing with atomic orbitals. It says that in a single atom no two electrons will have an identical set or the same quantum numbers (n, l, ml and ms). This means each electron in an atom has an unique configuration regarding quantum numbers and their orientation.
You have reached the end of Physics lesson 21.1.1 Background and Introduction to Quantum Numbers and Orbitals. There are 4 lessons in this physics tutorial covering Elementary Particles, you can access all the lessons from this tutorial below.
More Elementary Particles Lessons and Learning Resources
Whats next?
Enjoy the "Background and Introduction to Quantum Numbers and Orbitals" physics lesson? People who liked the "Elementary Particles lesson found the following resources useful:
- Background Feedback. Helps other - Leave a rating for this background (see below)
- Elementary Particles Physics tutorial: Elementary Particles. Read the Elementary Particles physics tutorial and build your physics knowledge of Elementary Particles
- Elementary Particles Revision Notes: Elementary Particles. Print the notes so you can revise the key points covered in the physics tutorial for Elementary Particles
- Elementary Particles Practice Questions: Elementary Particles. Test and improve your knowledge of Elementary Particles with example questins and answers
- Check your calculations for Elementary Particles questions with our excellent Elementary Particles calculators which contain full equations and calculations clearly displayed line by line. See the Elementary Particles Calculators by iCalculator™ below.
- Continuing learning elementary particles - read our next physics tutorial: Particles and Antiparticles - Interaction and Laws of Conservation
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Elementary Particles" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.