Menu
Physics Lesson 14.2.1 - The Experiment of Coulomb
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
Welcome to our Physics lesson on The Experiment of Coulomb, this is the first lesson of our suite of physics lessons covering the topic of Coulomb's Law, you can find links to the other lessons within this tutorial and access additional physics learning resources below this lesson.
The Experiment of Coulomb
Coulomb made an experiment using a torsion balance like the one shown in the figure below, which is an instrument used to measure small forces. It is based on the principle that a wire or thread resists twisting with a force that is proportional to the stress.
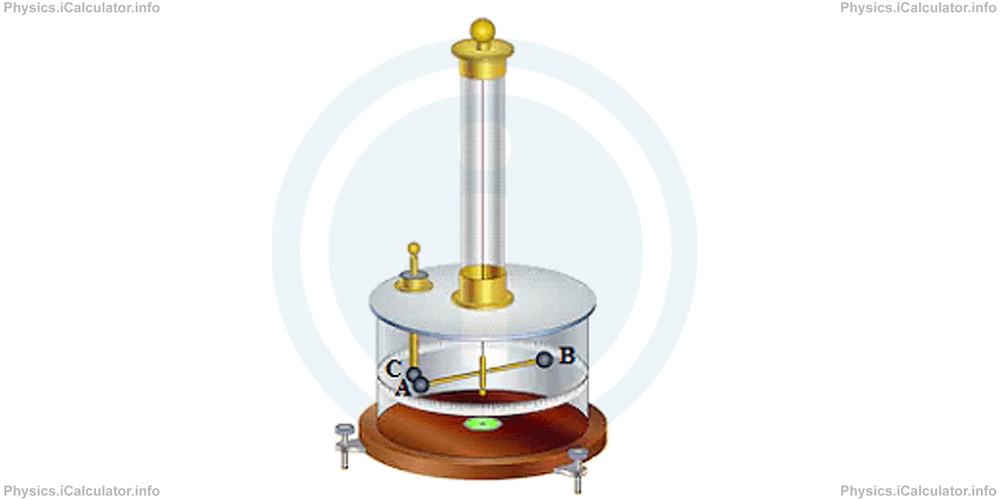
The apparatus contains three conducting spheres A, B and C where the spheres A and C are charged and the distance between them is adjustable. Obviously, they repel each other when they have the same charge. This causes a turning effect and as a result, the torsion balance rotates. Then, the angle of rotation - as dependent variable - is measured as a function of the amount of charge to determine the repulsive force.
Since it was impossible to measure too much accurately the angle of torsion in the times when Coulomb made this experiment, he used another technique, by charging the spheres A and C by different charges whose ratio is known. Thus, he discovered that if a charged sphere is brought in contact with an identical neutral sphere, the amount of charge in the first sphere is halved, i.e. it is shared equally between the two charges.
Then, Coulomb repeated this procedure with charges equal to half, quarter, etc., of the original amount and he thus was able to formulate the fundamental law of electrostatics for the point charges, as small charged spheres and point charges have the same behaviour. Coulomb discovered that:
- The magnitude of force between the charges is directly proportional to the product of magnitudes of charges |Q1| and |Q2| on both particles;
- The magnitude of force is inversely proportional to the product of distance between the charged particles;
- The force is attractive for unlike charges and repulsive for like ones; and
- The direction of force is along the line that joins the two charges.
Putting all together, Coulomb obtained the following equation:
where k is a constant of proportionality.
The above equation is the mathematical expression of Coulomb's Law, which states that:
The electrical force between two charged objects is directly proportional to the product of the quantity of charge on the objects and inversely proportional to the square of the separation distance between the two objects.
Electrical force keeps atoms together especially in solids.
Accurate measurements have shown that the value of constant k is
For simplicity, in most exercises we take k ≈ 9 × 109 N × m2 / C2.
Example 1
Two point charges of magnitudes Q1 = +3 μC and Q2 = - 4 μC are at 10 cm from each other. What is the electrostatic force between them? What conclusions do you draw from the sign of result?
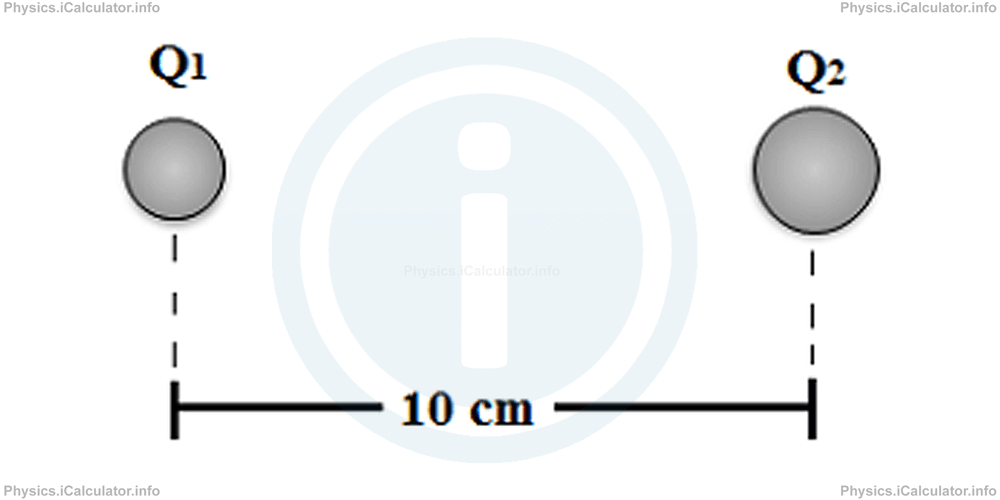
Solution 1
Applying the Coulomb's Law, we obtain after converting 10 cm = 10-1 m, 3 μC = 3 × 10-6 C and -4 μC = -4 × 10-6 C:
= 9 × 109 × 3 × 10-6 × (-4) × 10-6/(10-1 )2
= -10.8 N
The negative sign means the force produced is attractive as charges are of opposite type. If we choose one of the charges as origin (for example the first charge in the question above), the force vector acting on the other charge can express as
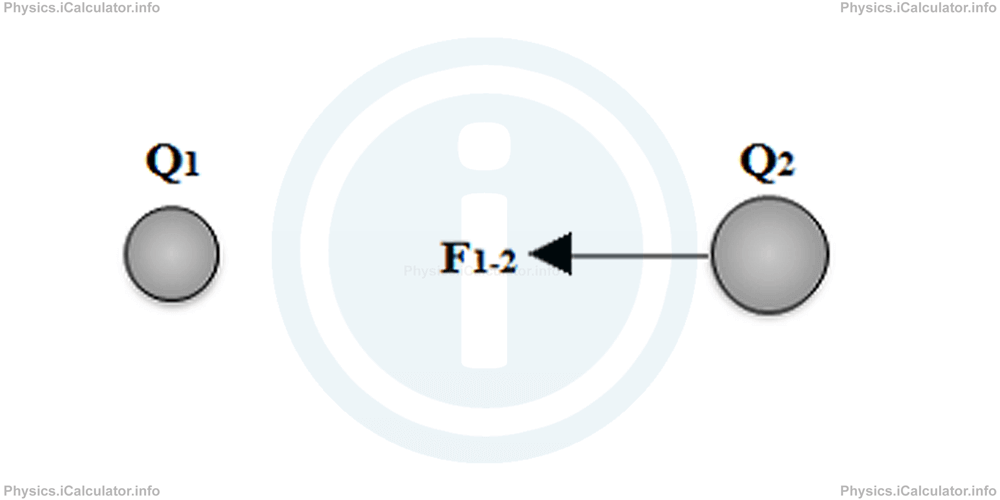
Finding the Point in which the Electrostatic Force is Zero
For this, we have to find an equilibrium point, in which the resultant force is zero. We have to take a trial charge (usually positive) Q0 and use it to find the equilibrium position.
Let's assume the charges Q1 and Q2 are at a distance r from each other and the trial charge Q0 is somewhere in between, at a distance d from the first charge. Obviously, the distance of the trial charge Q0 from the second charge Q2 is r - d. Thus, we can write for the forces in the equilibrium position
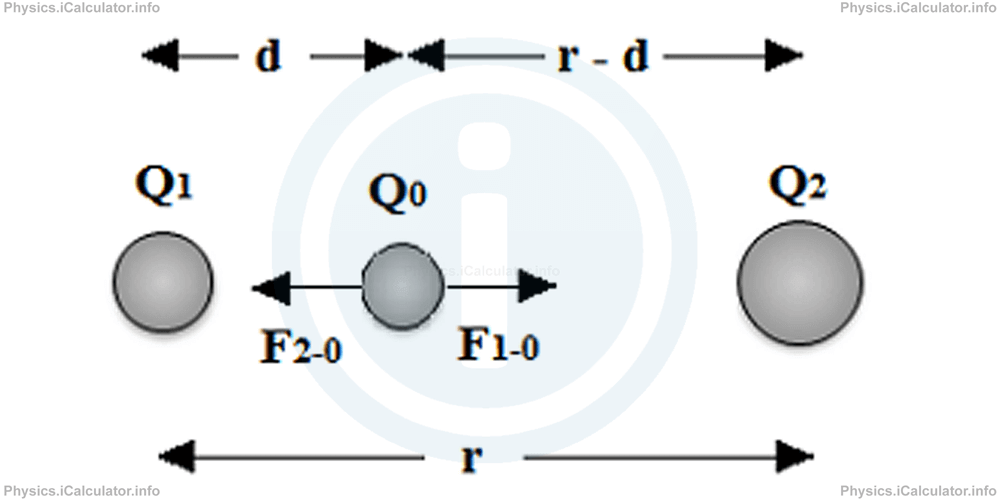
From Coulomb's Law, we have for the forces acting on the trial charge Q0:
where F1-0 and F2-0 are the forces applied by the charges Q1 and Q2 on the trial charge Q0. Therefore, we obtain:
After simplifications, we obtain
Example 2
Two charges Q1 = 3 C and Q2 = 12 C are 0.5 m away from each other. What is the distance from the first object in which the resultant electrostatic force is zero?
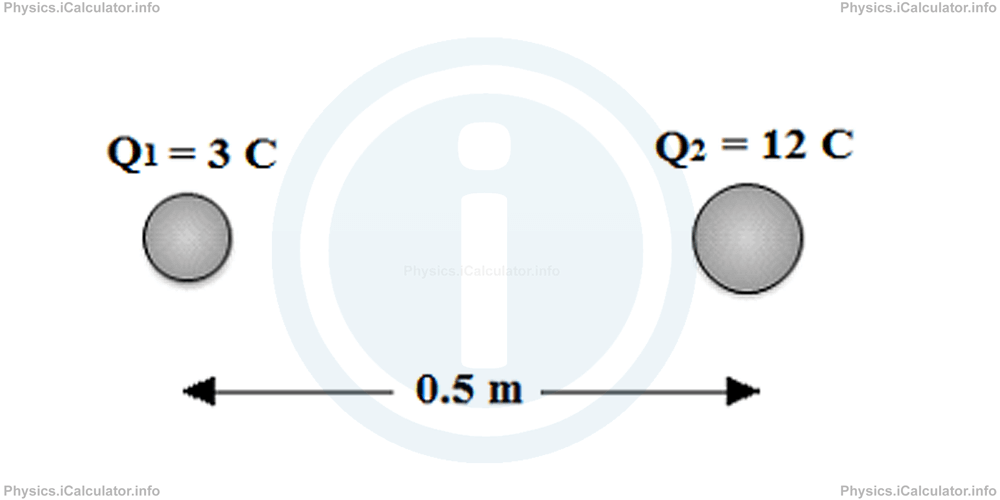
Solution 2
If we denote by d the required distance from the charge Q1, we obtain
3/d2 = 12/(0.5 - d)2
1/d2 = 4/(0.5 - d)2
4 × d2 = (0.5-d)2
4 × d2 = 0.52 - 2 × 0.5 × d + d2
4d2 = 0.25 - d + d2
3d2 + d-0.25 = 0
Solving this quadratic equation, we obtain d = 1/6 m.
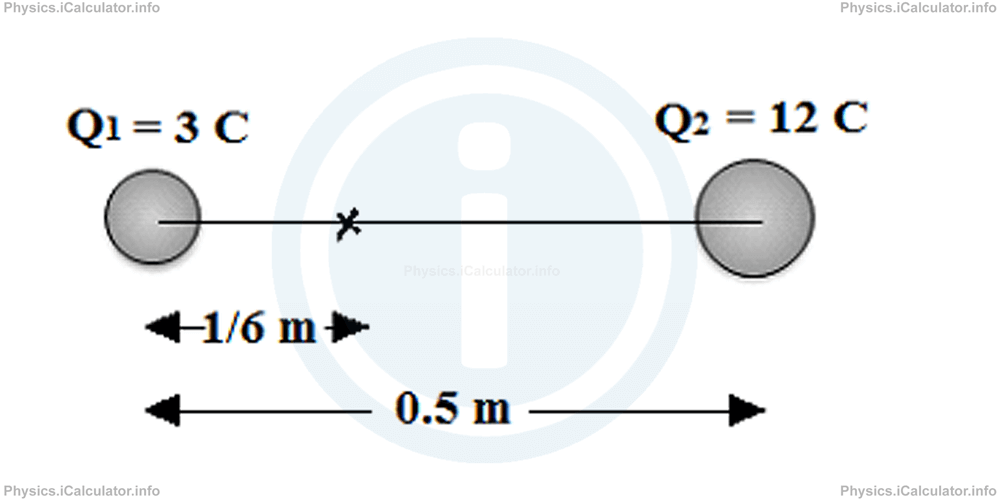
When charges are of opposite sign, we expect the equilibrium position be in the direction of the smallest charge, not in the line that connects the two charges. Not always, this is possible (i.e. to find the equilibrium position when the charges are of opposite sign). Let's consider an example.
Example 3
Two electric charges Q1 = 1°C and Q2 = -1 C are 20 cm away from each other. Find the point (if any) in which the resultant electrostatic force is zero.
Solution 3
The only possibility to find an equilibrium point is to have it on the right of Q2 as if we consider the positive point charge discussed earlier, it is repelled by Q1 and attracted by Q2. This equilibrium point must be closer to the small charge. Thus, if we denote by d the distance from Q2 to equilibrium position, the distance from Q1 to this point is r + d. Therefore, giving that r = 20 cm = 0.2 m, we have:
Substituting the known values, we obtain
(0.08 + d)2 = -10 d2
0.0064 + 0.16 d + d2 = -10 d2
11d2 + 0.16 d + 0.0064 = 0
This quadratic equation has no roots, as its discriminant is negative. This means there is not any point in which the resultant electrostatic force is zero.
You have reached the end of Physics lesson 14.2.1 The Experiment of Coulomb. There are 2 lessons in this physics tutorial covering Coulomb's Law, you can access all the lessons from this tutorial below.
More Coulomb's Law Lessons and Learning Resources
Whats next?
Enjoy the "The Experiment of Coulomb" physics lesson? People who liked the "Coulomb's Law lesson found the following resources useful:
- Experiment Feedback. Helps other - Leave a rating for this experiment (see below)
- Electrostatics Physics tutorial: Coulomb's Law. Read the Coulomb's Law physics tutorial and build your physics knowledge of Electrostatics
- Electrostatics Revision Notes: Coulomb's Law. Print the notes so you can revise the key points covered in the physics tutorial for Coulomb's Law
- Electrostatics Practice Questions: Coulomb's Law. Test and improve your knowledge of Coulomb's Law with example questins and answers
- Check your calculations for Electrostatics questions with our excellent Electrostatics calculators which contain full equations and calculations clearly displayed line by line. See the Electrostatics Calculators by iCalculator™ below.
- Continuing learning electrostatics - read our next physics tutorial: Electric Field
Help others Learning Physics just like you
Please provide a rating, it takes seconds and helps us to keep this resource free for all to use
We hope you found this Physics lesson "Coulomb's Law" useful. If you did it would be great if you could spare the time to rate this physics lesson (simply click on the number of stars that match your assessment of this physics learning aide) and/or share on social media, this helps us identify popular tutorials and calculators and expand our free learning resources to support our users around the world have free access to expand their knowledge of physics and other disciplines.